Utilizing Advanced Technologies to Augment Pharmacovigilance Systems: Challenges and Opportunities | Therapeutic Innovation & Regulatory Science
![Martín Cañás on Twitter: "Sistemas de farmacovigilancia en los países en desarrollo Revisión sistemática utilizando los indicadores de farmacovigilancia de la OMS [Ther Innov Regul Sci] https://t.co/MPDWLoOXYE https://t.co/VvNl5VMYC7" / Twitter Martín Cañás on Twitter: "Sistemas de farmacovigilancia en los países en desarrollo Revisión sistemática utilizando los indicadores de farmacovigilancia de la OMS [Ther Innov Regul Sci] https://t.co/MPDWLoOXYE https://t.co/VvNl5VMYC7" / Twitter](https://pbs.twimg.com/media/FyqhkqLXwAEE0_2.jpg)
Martín Cañás on Twitter: "Sistemas de farmacovigilancia en los países en desarrollo Revisión sistemática utilizando los indicadores de farmacovigilancia de la OMS [Ther Innov Regul Sci] https://t.co/MPDWLoOXYE https://t.co/VvNl5VMYC7" / Twitter
Reducing Uninformative IND Safety Reports: A List of Serious Adverse Events anticipated to Occur in Patients with Lung Cancer

PDF) A Comparative Review of Waivers Granted in Pediatric Drug Development by FDA and EMA from 2007-2013

A systematic review of models of patient engagement in the development and life cycle management of medicines - ScienceDirect
BACKGROUND METHODS RESULTS Michael DeLuca,1 Evelyn Hermes-DeSantis,2 and Keyur Brahmbhatt 3 CONCLUSIONS

PDF) Quantitative Benefit-Risk Assessment: State of the Practice Within Industry Quantitative Benefit-Risk Assessment: State of the Practice Within Industry

Applied Sciences | Free Full-Text | Development of a Mobile Application for Smart Clinical Trial Subject Data Collection and Management
![Martín Cañás on Twitter: "Sistemas de farmacovigilancia en los países en desarrollo Revisión sistemática utilizando los indicadores de farmacovigilancia de la OMS [Ther Innov Regul Sci] https://t.co/MPDWLoOXYE https://t.co/VvNl5VMYC7" / Twitter Martín Cañás on Twitter: "Sistemas de farmacovigilancia en los países en desarrollo Revisión sistemática utilizando los indicadores de farmacovigilancia de la OMS [Ther Innov Regul Sci] https://t.co/MPDWLoOXYE https://t.co/VvNl5VMYC7" / Twitter](https://pbs.twimg.com/media/FUpi66gWUAAfRzm.jpg)
Martín Cañás on Twitter: "Sistemas de farmacovigilancia en los países en desarrollo Revisión sistemática utilizando los indicadores de farmacovigilancia de la OMS [Ther Innov Regul Sci] https://t.co/MPDWLoOXYE https://t.co/VvNl5VMYC7" / Twitter
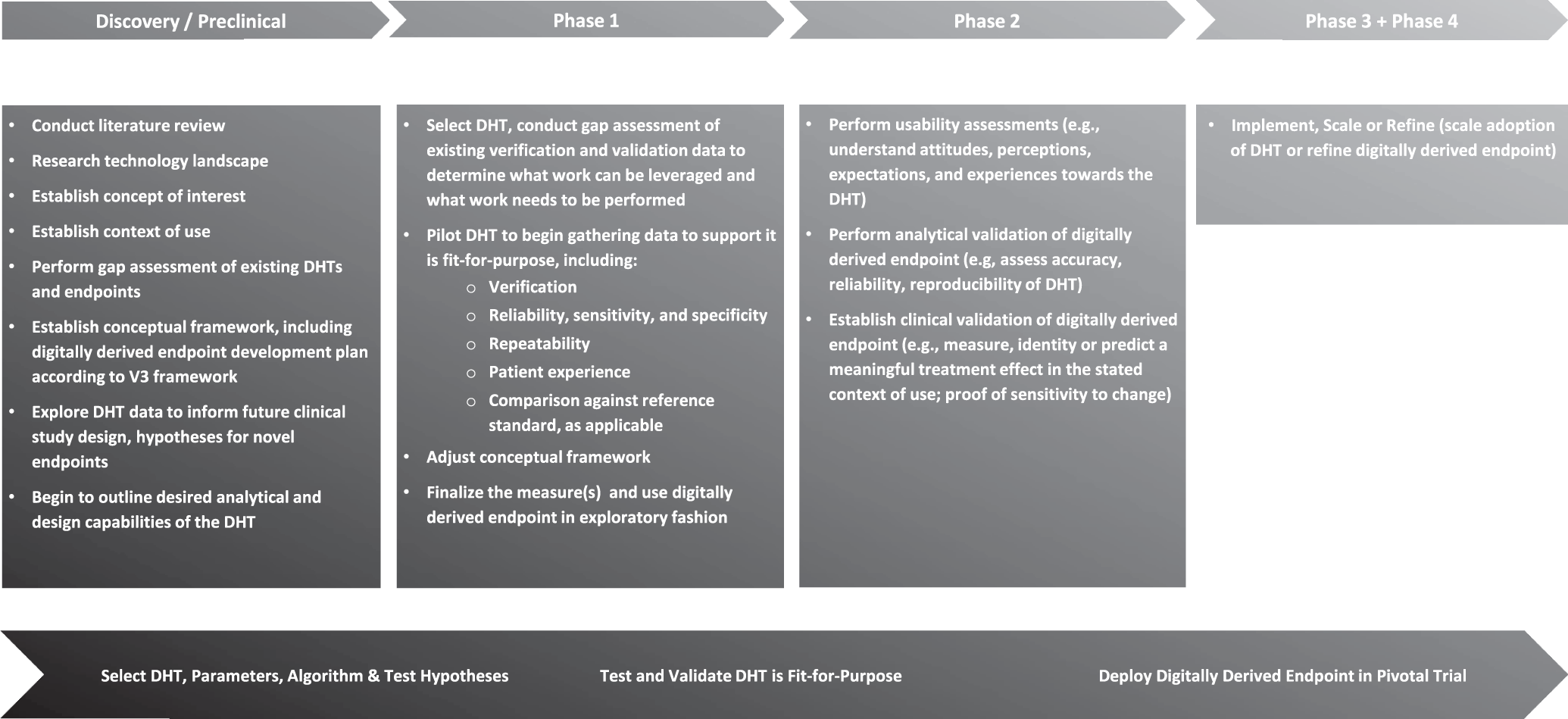
Incorporating digitally derived endpoints within clinical development programs by leveraging prior work | npj Digital Medicine
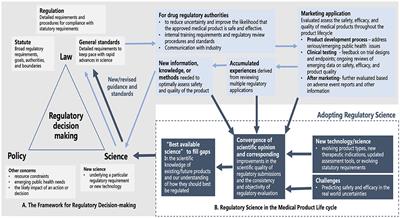
Frontiers | Application of implementation science framework to develop and adopt regulatory science in different national regulatory authorities

Bringing Patient and Caregivers Voices to the Clinical Trial Chorus: A Report From the BMT CTN Patient and Caregiver Advocacy Task Force - Transplantation and Cellular Therapy, Official Publication of the American

El empoderamiento de los pacientes con enfermedades raras y su creciente incorporación en la toma de decisiones - NewsRARE