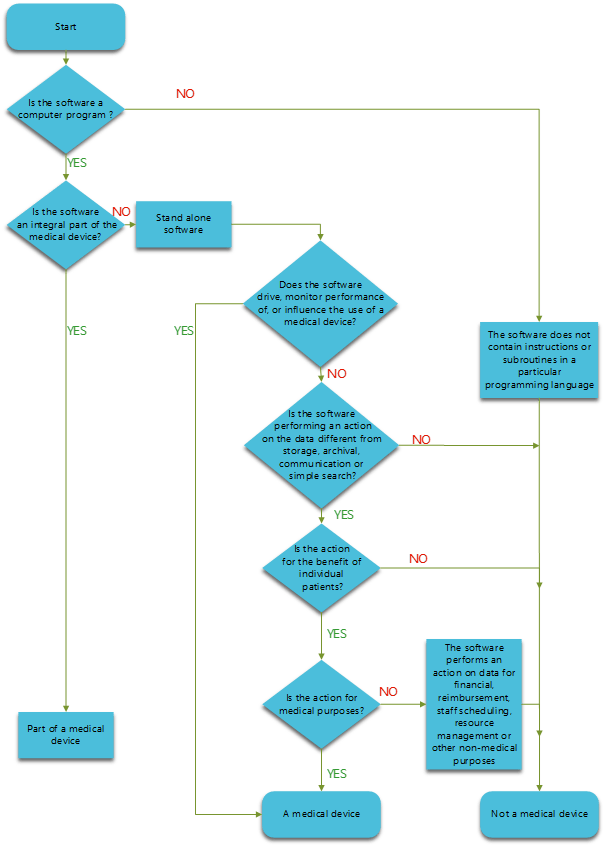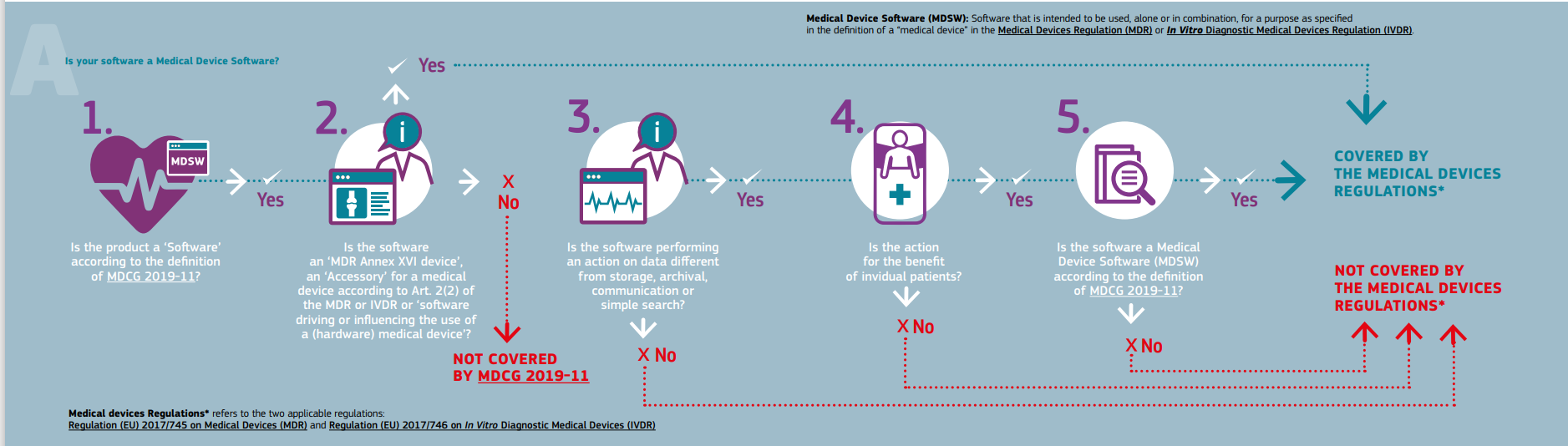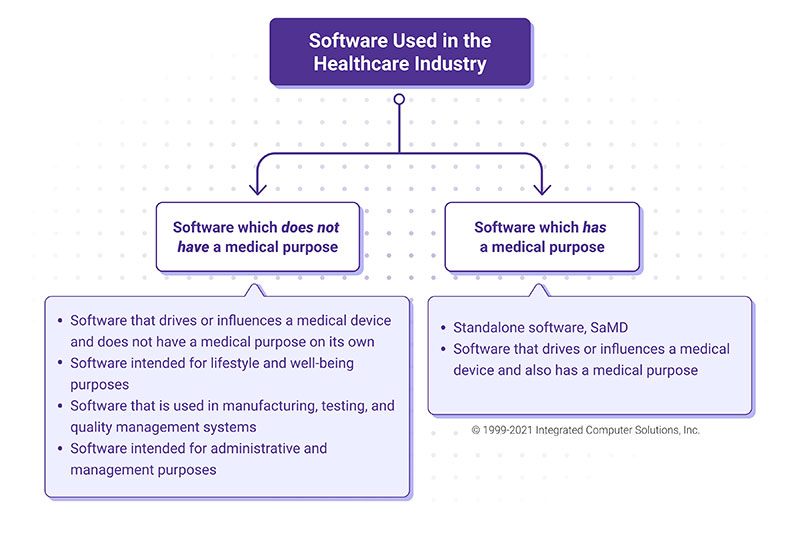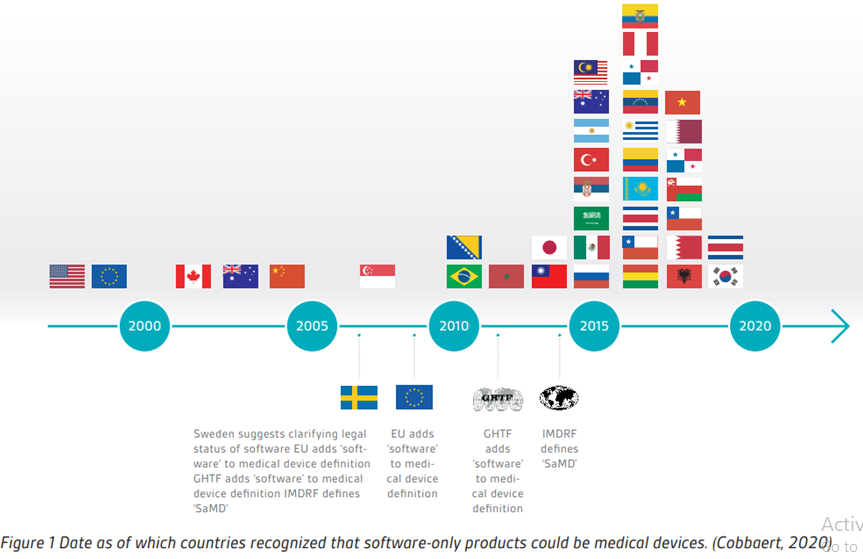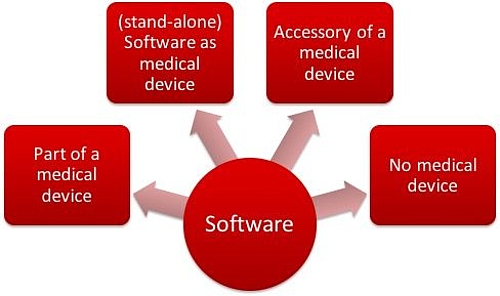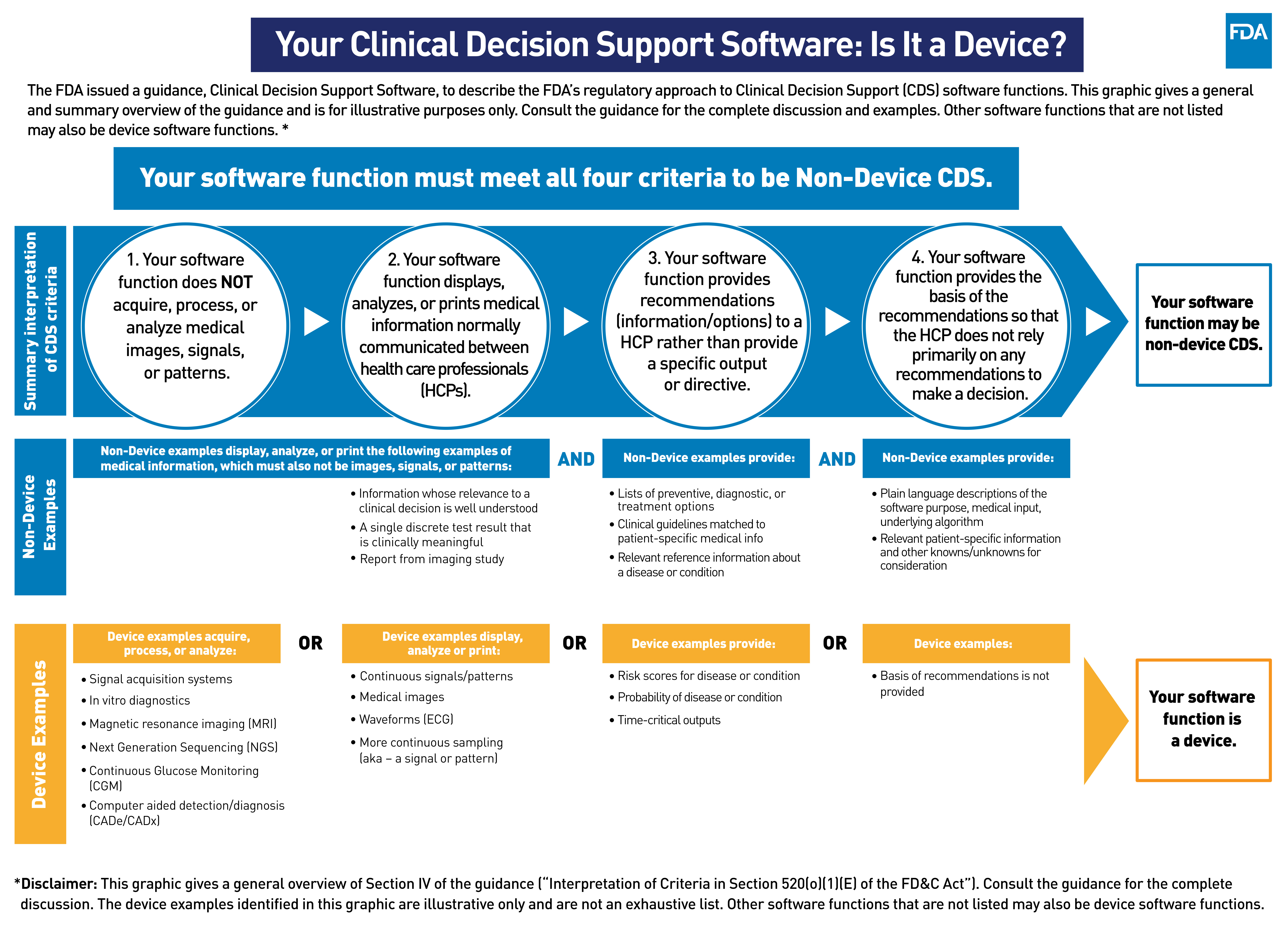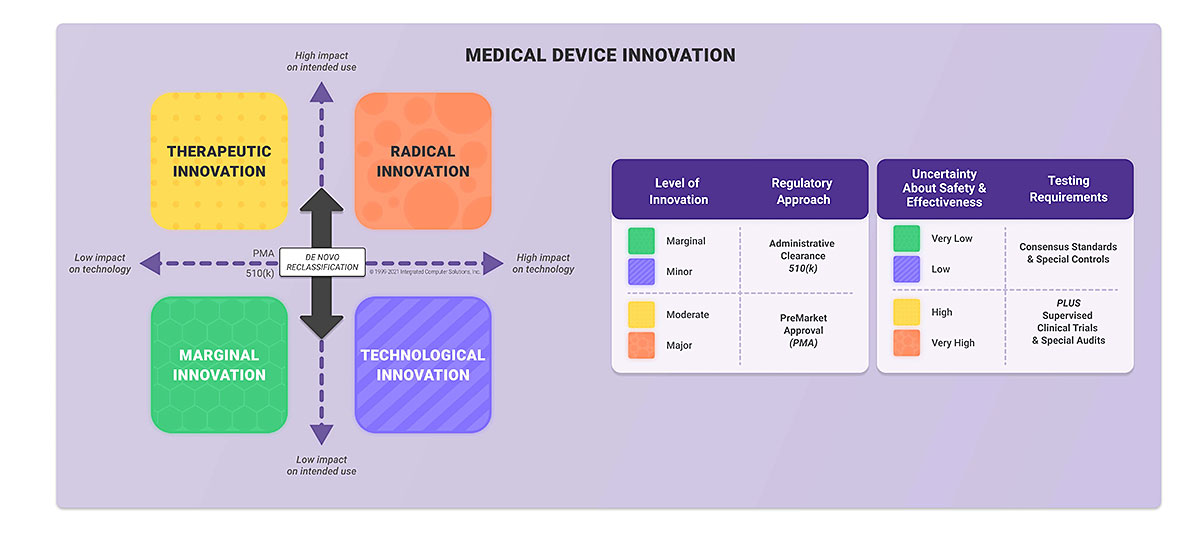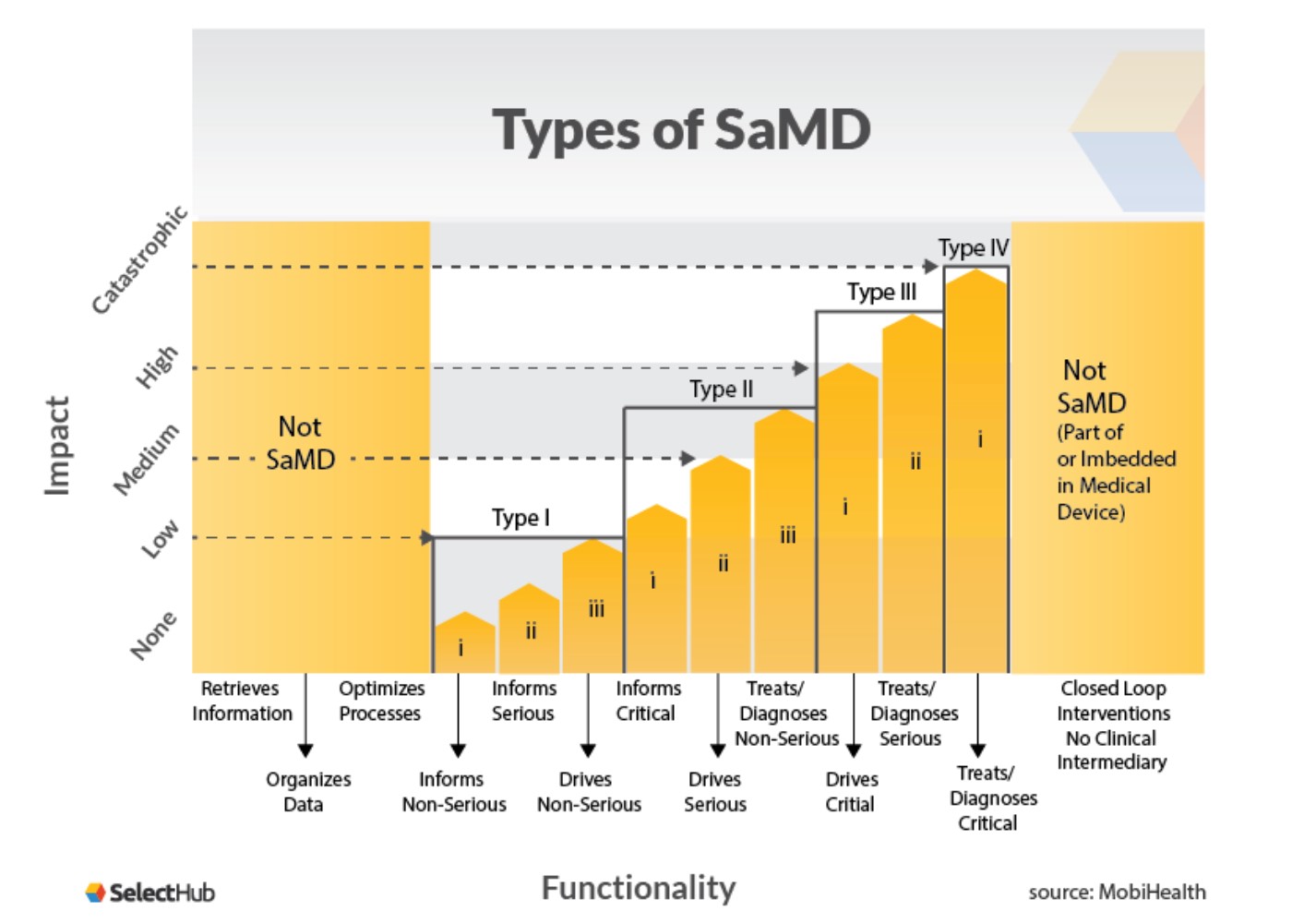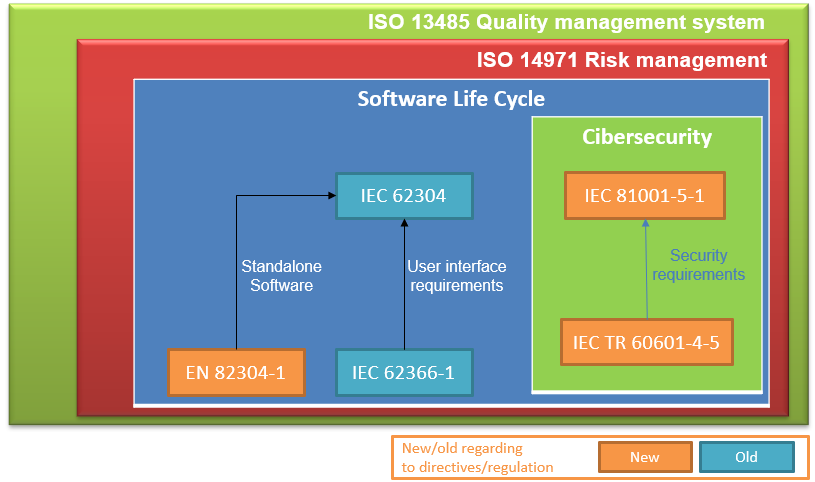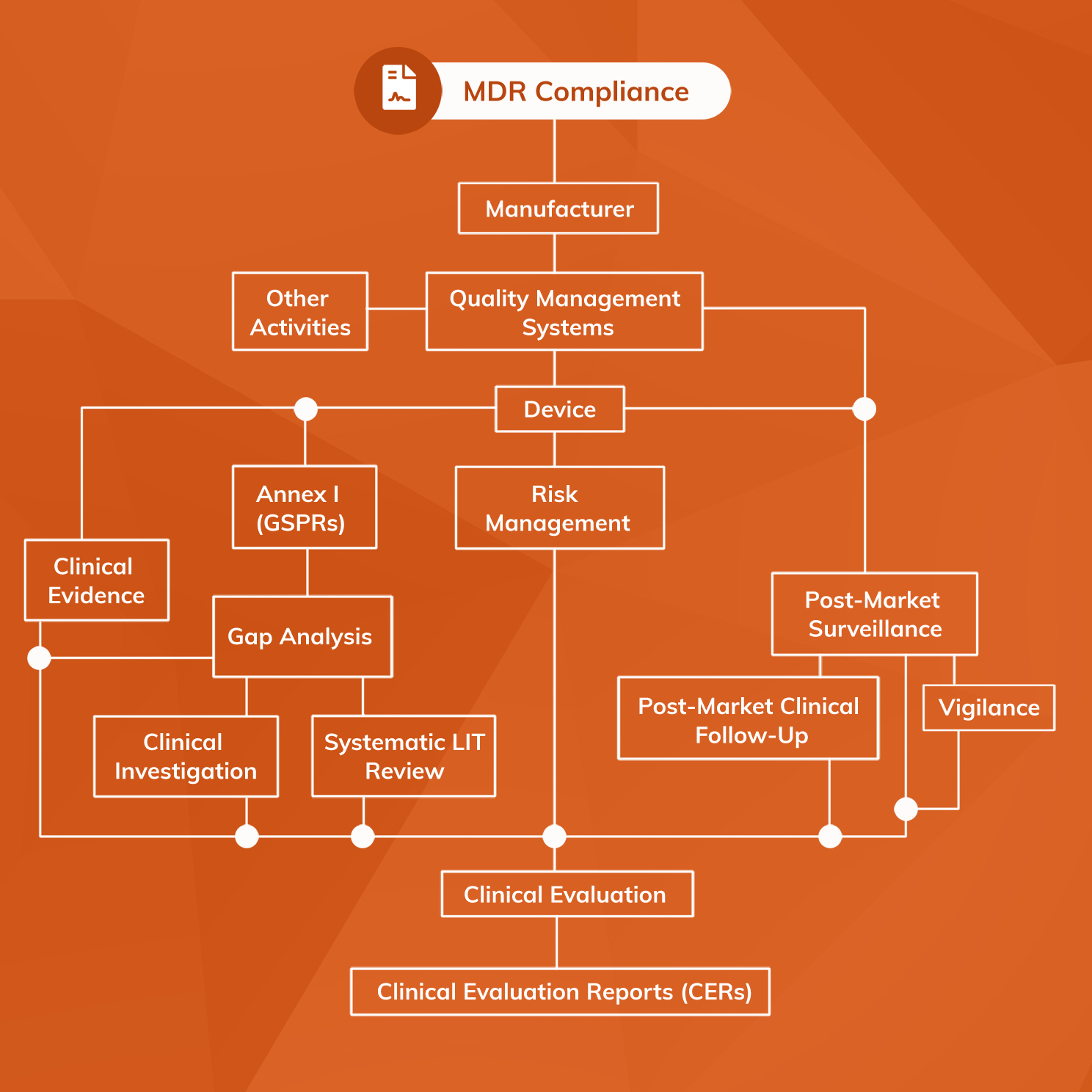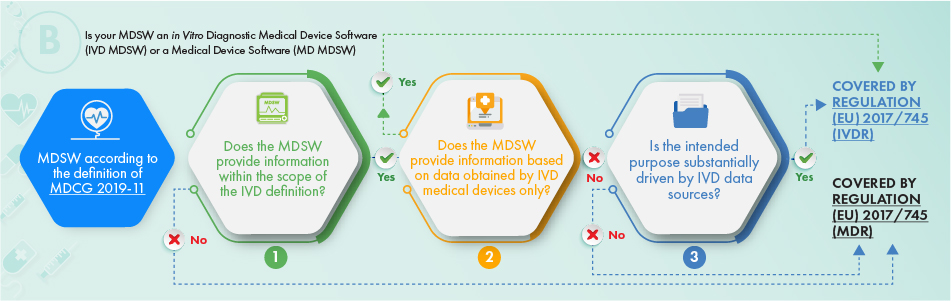
MDCG Guidance for Medical Device Software | Freyr - Global Regulatory Solutions and Services Company

BHHSERIES- MEDICAL DEVICE WEBINAR 'Medical Device Regulation (#MDR) for medical software: Are you ready?' - Biotech Spain
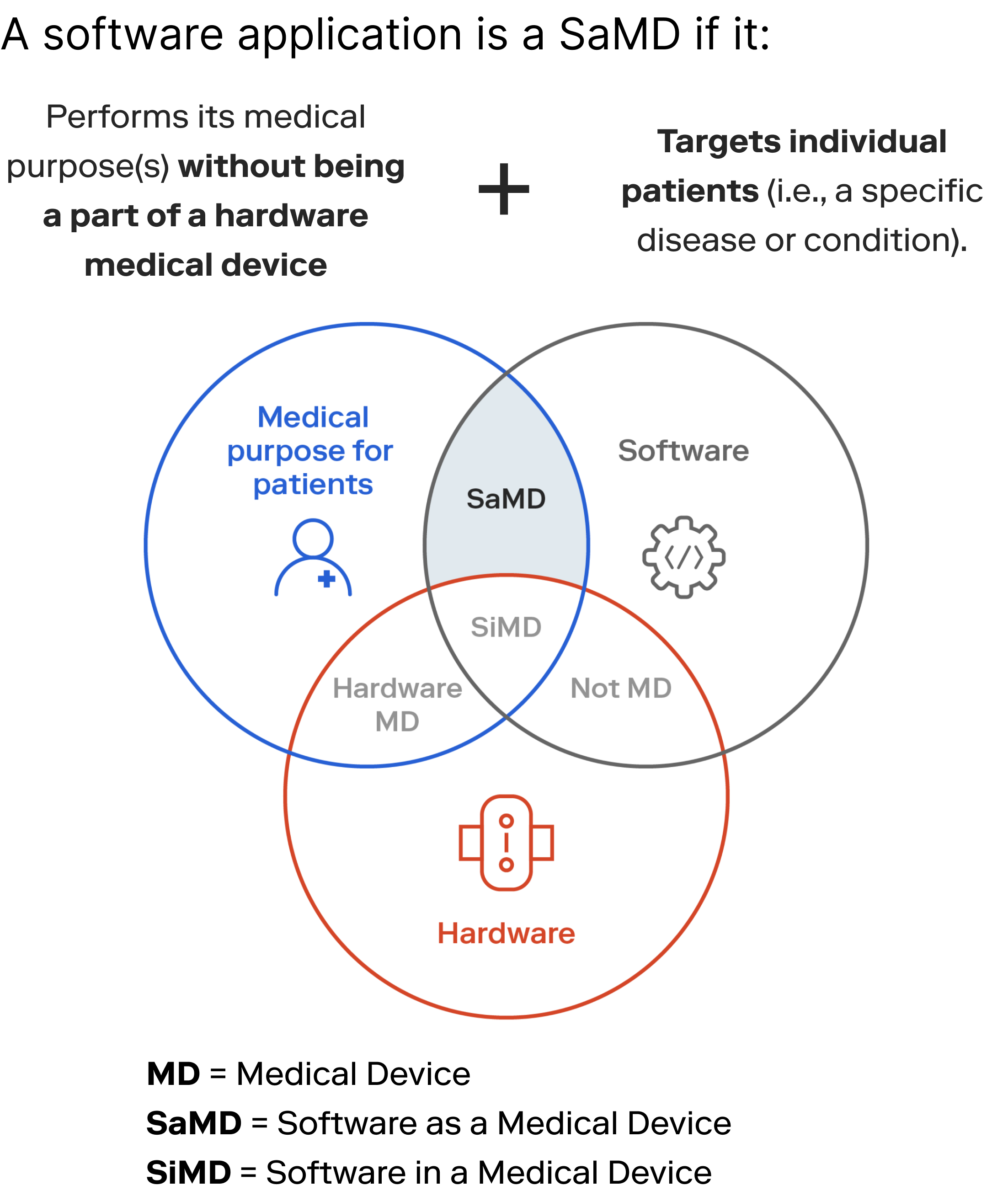
What is Software as a Medical Device? A guide to building products & platforms at the speed of tech, not healthcare
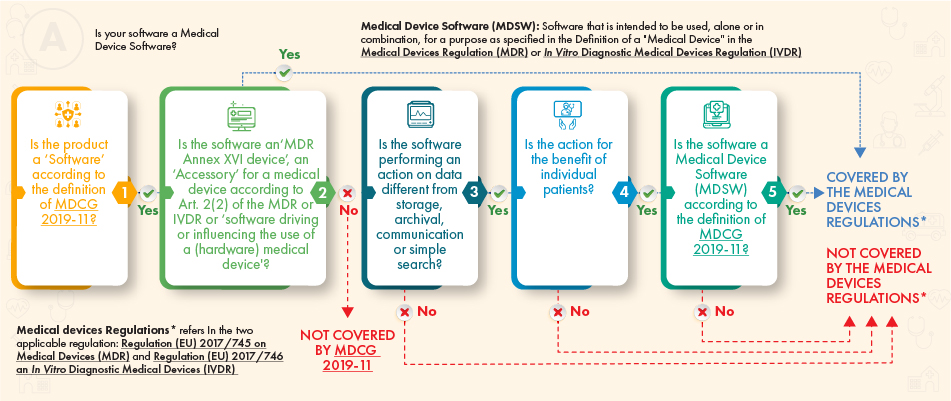
MDCG Guidance for Medical Device Software | Freyr - Global Regulatory Solutions and Services Company