Solve Expiry Labels, DtP, and Timelines for EU 536/2014 Clinical Trials Regulation | Healthcare Packaging
The new Clinical Trial Regulation (536/2014): moving towards implementation. Overview of progress so far

Clinical Trial Regulation 536/2014 – TEDDY – European Network of Excellence for Paediatric Clinical Research

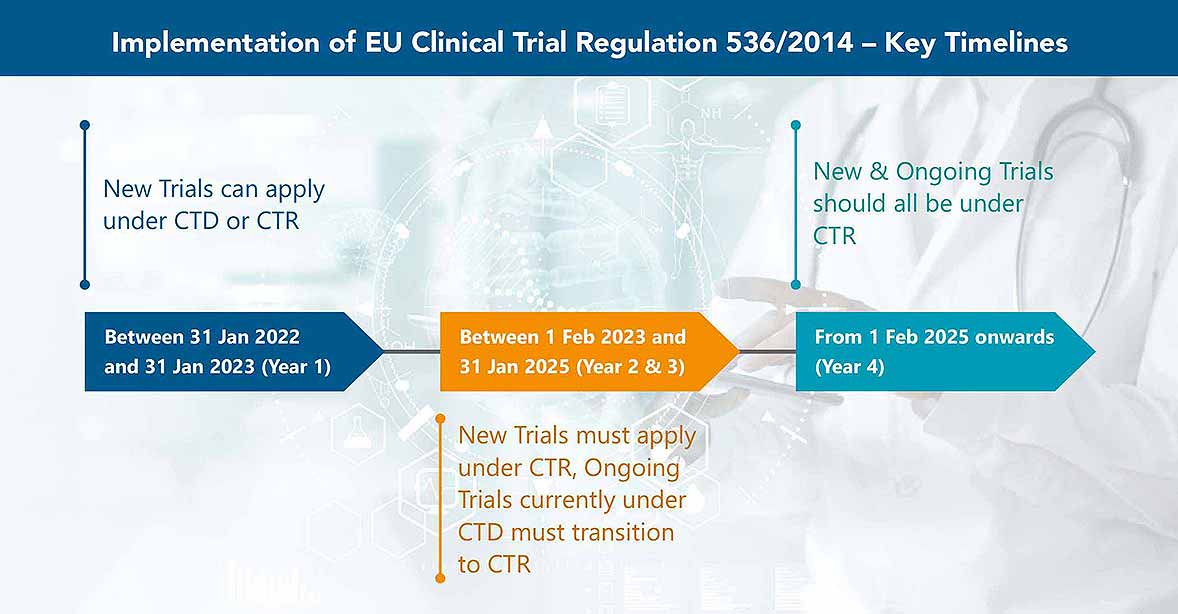
New | The Regulation (EU) 536/2014 comes into force mandatorily from today for all clinical trials to be initiated!

Quick guide on the rules and procedures of the EU Clinical Trials Regulation drawn up by the Clinical Trials Coordination and Advisory Group (CTAG) - Formiventos

ECCRT on Twitter: "Join our webinar to learn about scope, definitions, application and validation processes, mandatory notifications, rules for safety reporting, timelines and time table for implementation of the European Clinical Trial