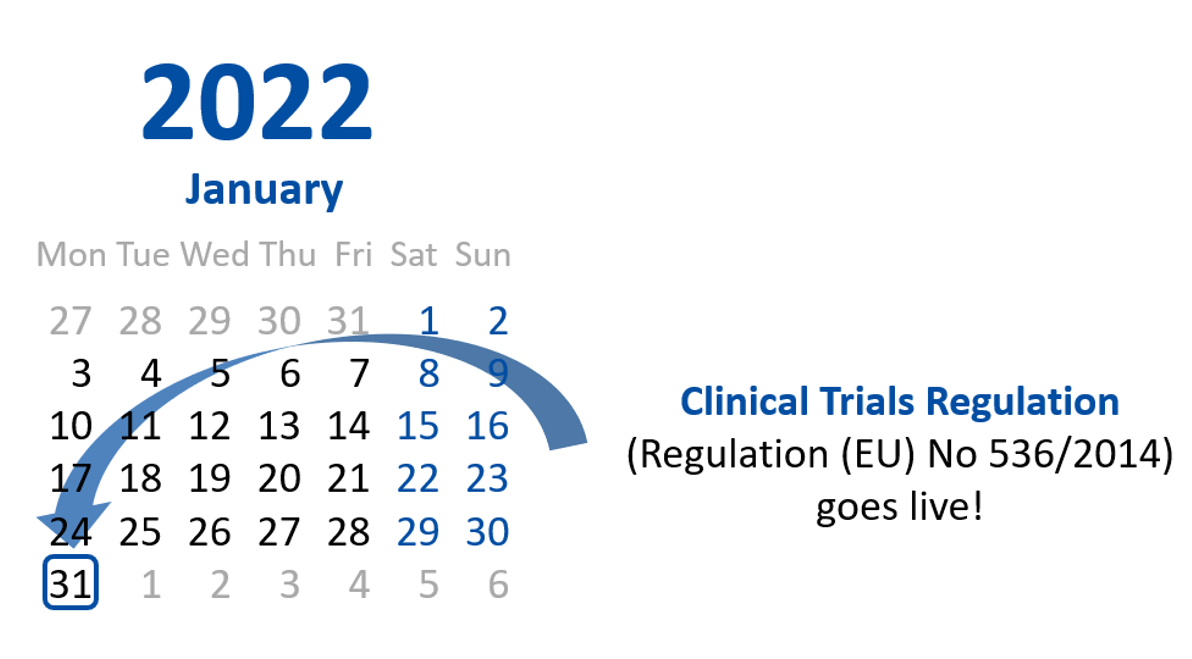
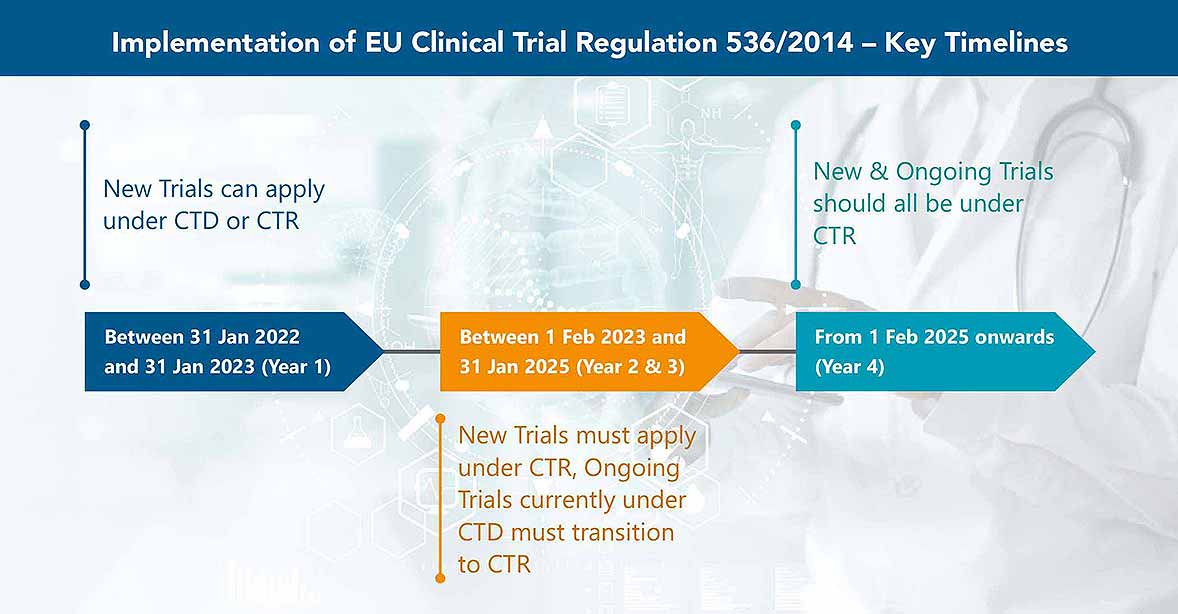
New | The Regulation (EU) 536/2014 comes into force mandatorily from today for all clinical trials to be initiated!
September 2021 The rules governing medicinal products in the European Union VOLUME 10 - Guidance documents applying to clinical

ECCRT on Twitter: "Join our webinar to learn about scope, definitions, application and validation processes, mandatory notifications, rules for safety reporting, timelines and time table for implementation of the European Clinical Trial