European Parliament resolution of 23 June 2022 on Commission Implementing Decision (EU) 2022/797 of 19 May 2022 authorising the

The Right to Adopt Post-Market Restrictions of Genetically Modified Crops in the EU – A Shift from De-Centralised Multi-Level to Centralised Governance in the Case of GM Foods | European Journal of
SCIENTIFIC OPINION Opinion on application reference EFSA-GMO-RX-Bt11 for renewal of the authorisation of existing products produ
European Parliament resolution of 31 January 2019 on the draft Commission implementing decision authorising the placing on the m
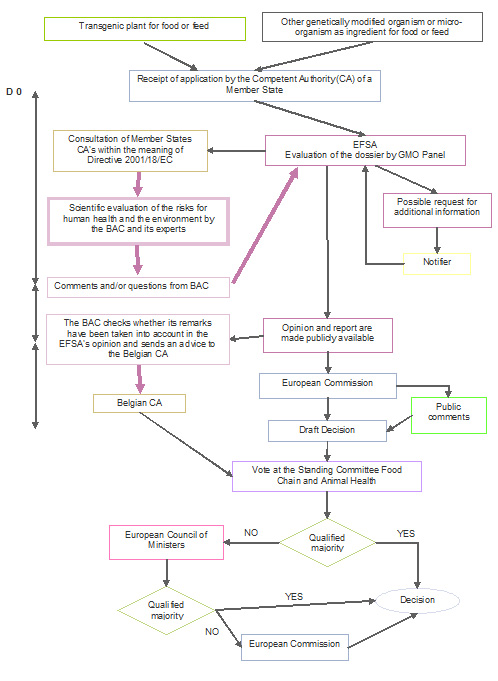
Regulation (EC) No 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and
The regulatory status of honey with respect of the Novel Food Regulation (EC) No 258/97 has been discussed at the Standing Com
Assessment of genetically modified soybean MON 89788 for renewal of authorisation under Regulation (EC) No 1829/2003 (applica
▻B REGULATION (EC) No 1829/2003 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 22 September 2003 on genetically modified foo

PPT - EU NEW GMO LEGISLATION Regulation 1829/2003 Regulation 1830/2003 PowerPoint Presentation - ID:6806665

PDF) Guidance for renewal applications of genetically modified food and feed authorised under Regulation (EC) No 1829/2003
EUROPEAN COMMISSION Standing Committee on Plants, Animals, Food and Feed Section Genetically Modified Food and Feed 21 February
Overall opinion of the European Food Safety Authority in accordance with Articles 6 and 18 of Regulation (EC) No 1829/2003 on ap
PRODUCT SPECIFICATIONS 080008462 WHITE CHOCOLATE EDELWEISS KG.15 Dimensional characteristics Storage & Shelf life Compositio

PDF) Statement on a request from the European Commission related to the emergency measure notified by Bulgaria on genetically modified maize MON 810 according to Article 34 of Regulation (EC) 1829/2003
EFSA guidance on the submission of applications for authorisation of genetically modified plants under Regulation (EC) No 1829/2