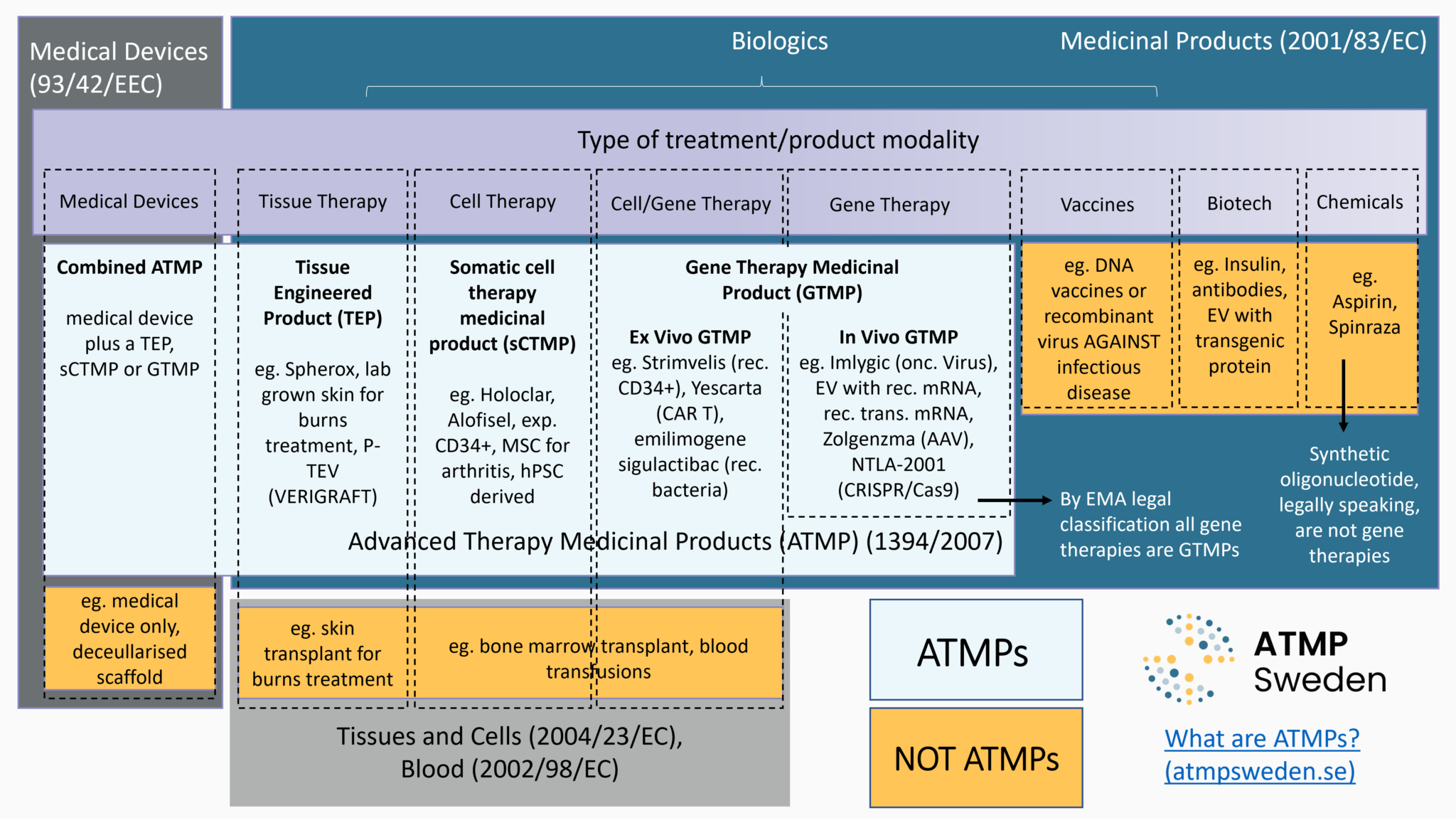
Advanced Therapy Medicinal Products for the Eye: Definitions and Regulatory Framework. - Abstract - Europe PMC

Retrovirus mediated hematopoietic gene therapy: A European regulatory perspective with special focus on the situation in Germany
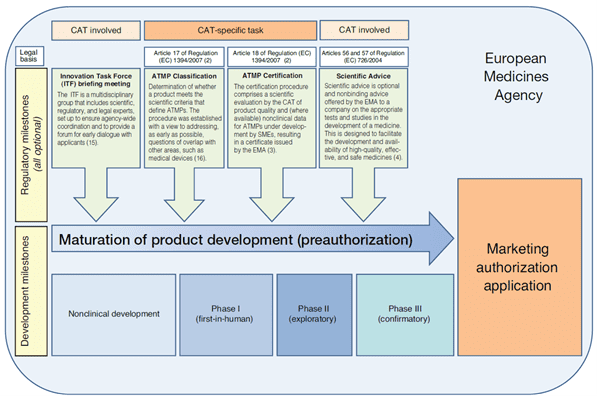
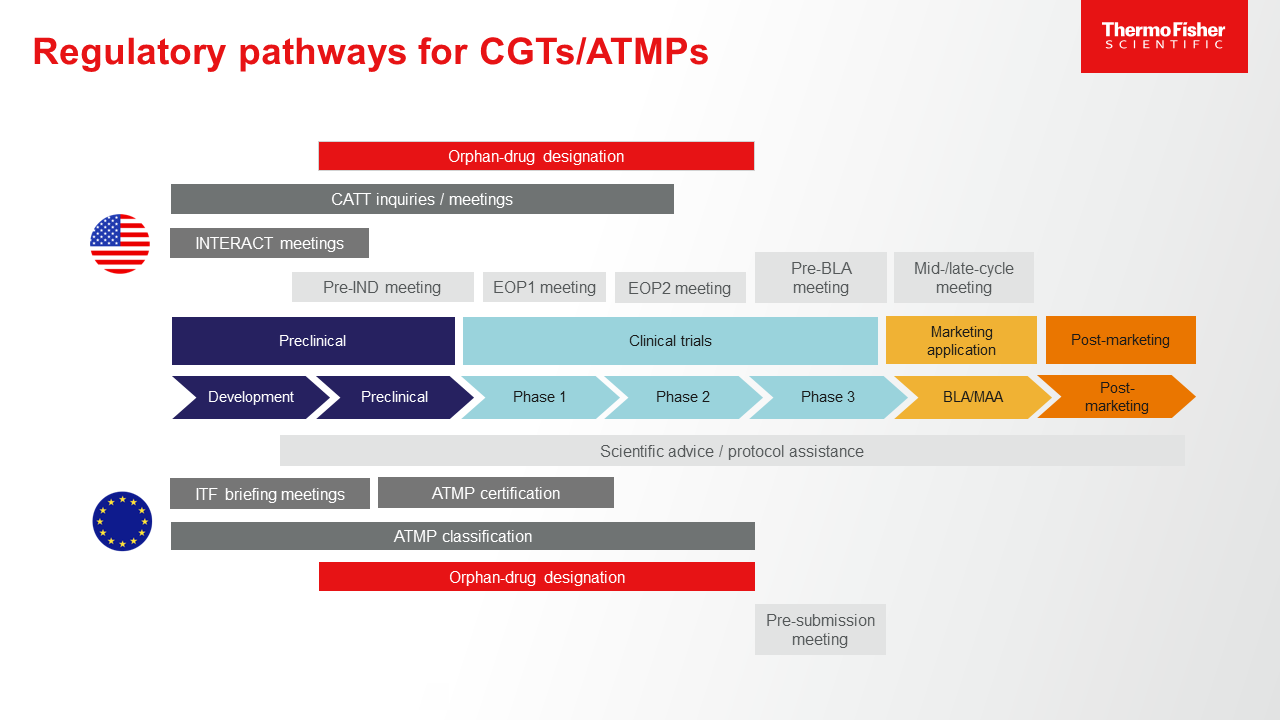
How to design a regulatory strategy to optimize registration of Advanced Therapy Medicinal Products (ATMPs) with European Medicines Agency ? - BlueReg Group

IIS Biodonostia on Twitter: "🟩 Iraida Loinaz de @CIDETEC_ toma la palabra en la ponencia titulada “Vectores no virales para terapias avanzadas” @uik_eus @DonostiakoOsp @osakidetzaEJGV #innovación #salud #Ikerketaosasunean #TerapiasAvanzadas ...
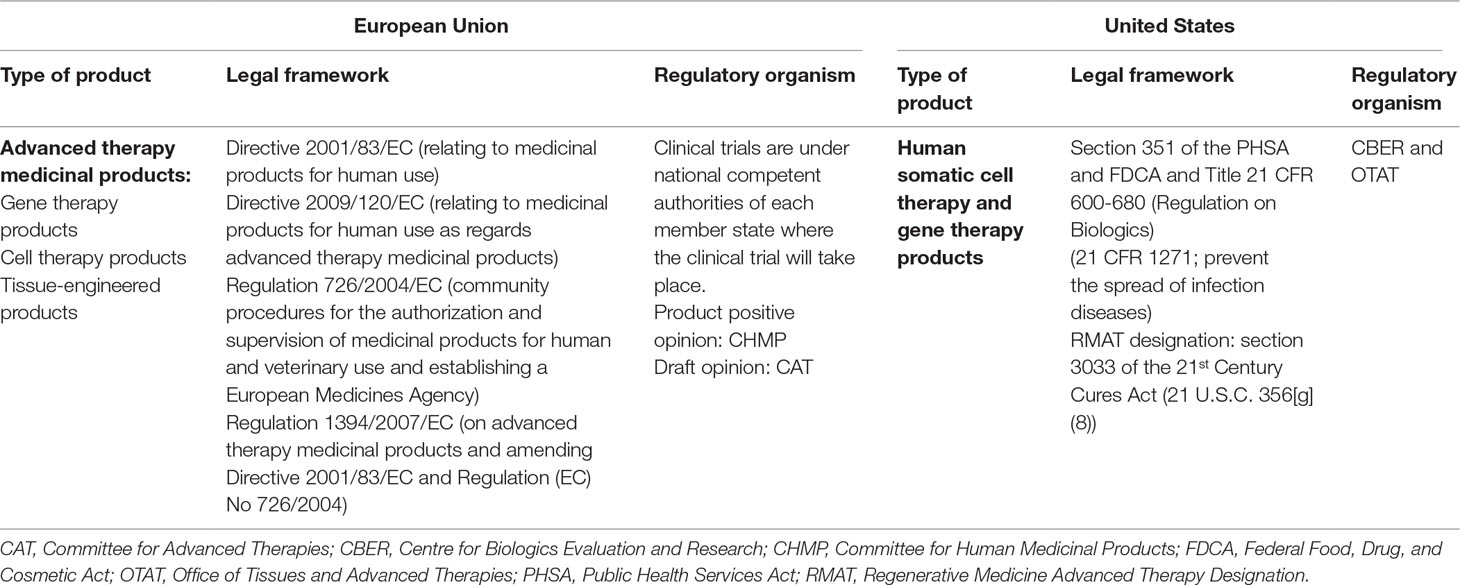
Frontiers | Regulatory Framework for Advanced Therapy Medicinal Products in Europe and United States
LEGISLATION AND GUIDELINES 1 PURPOSE 2 SCOPE 3 EU LEGISLATION 3.1 Regulations 3.2 Directives 3.3 Guidances and guidelines
REGULATION (EC) No 1394/2007 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 13 November 2007 on advanced therapy medicinal pro