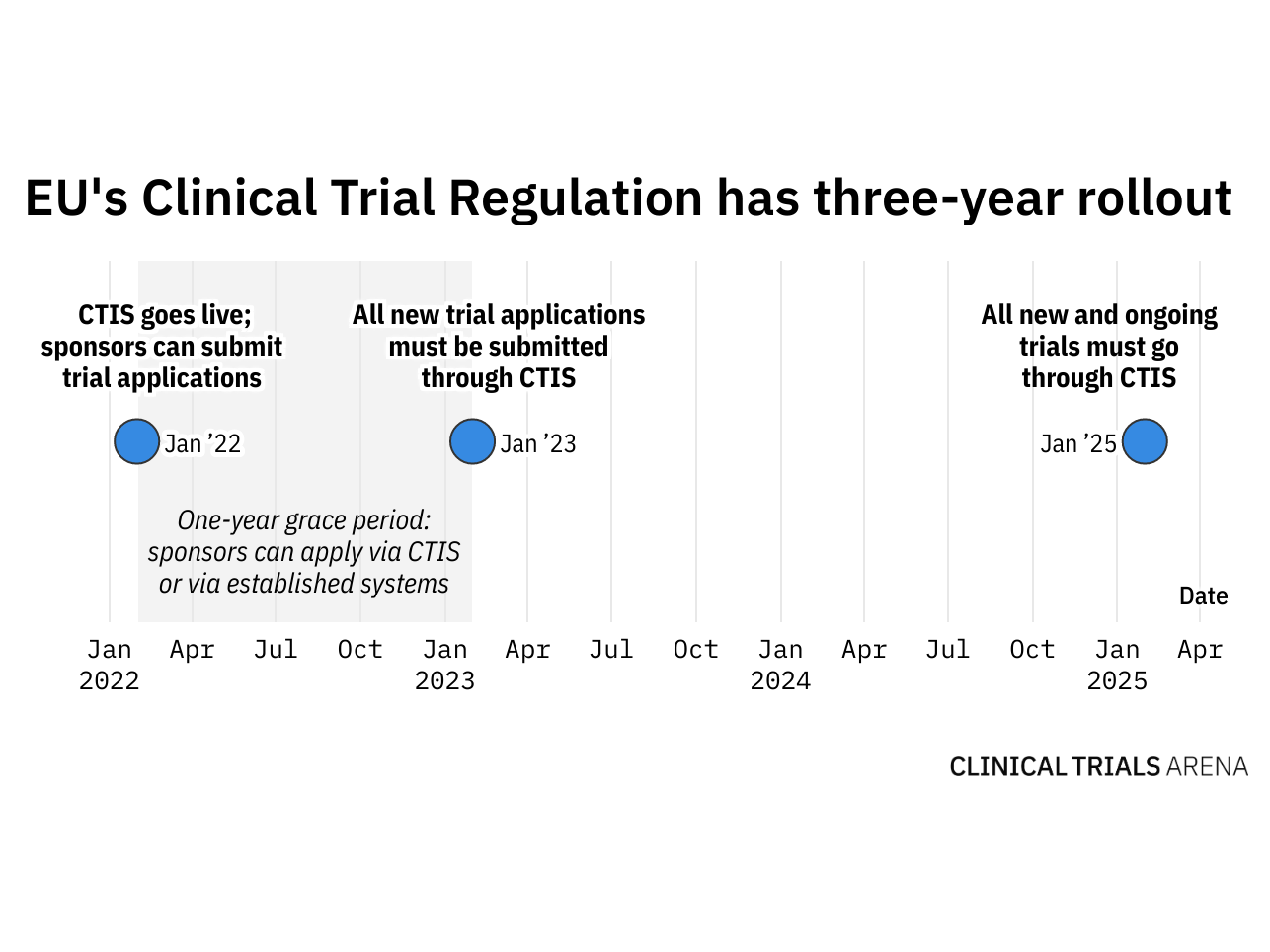
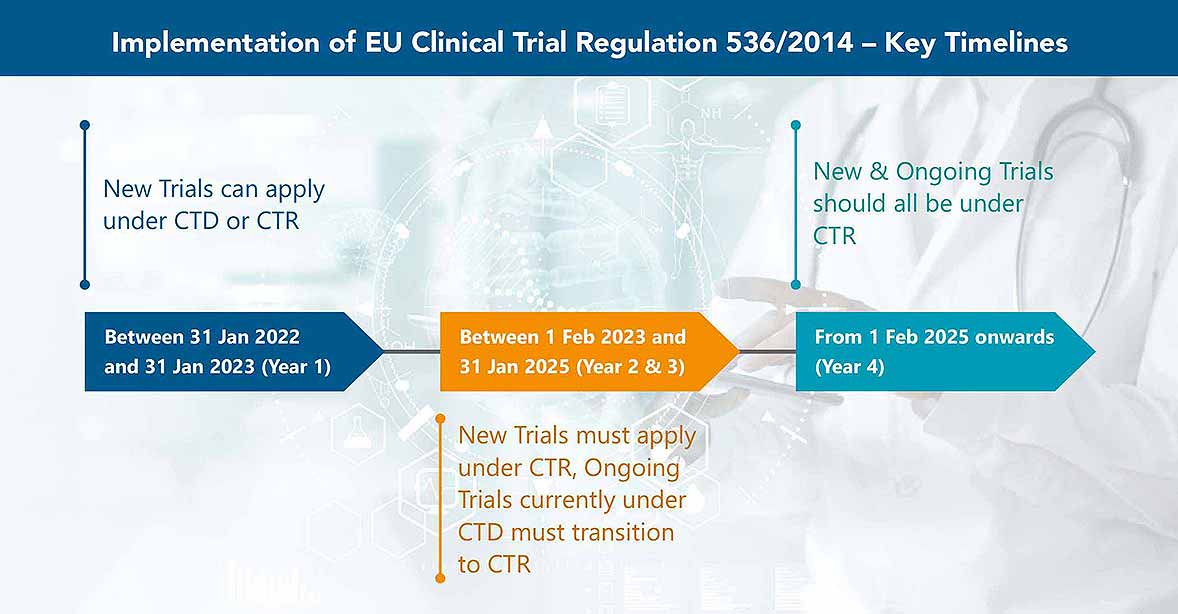
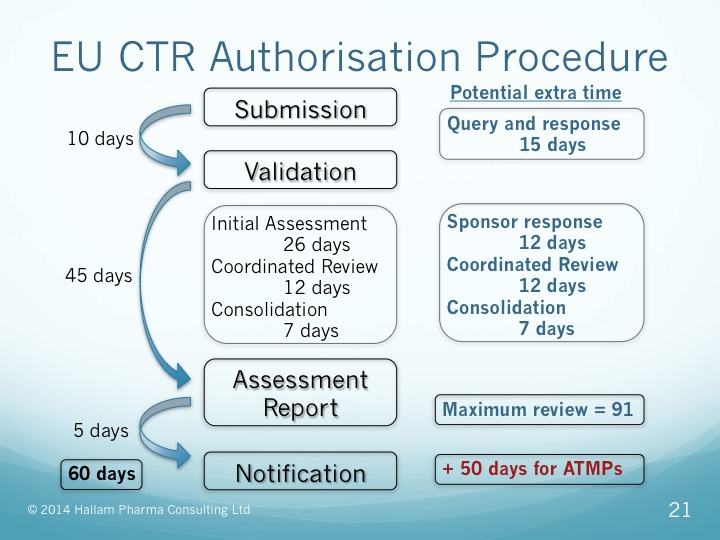
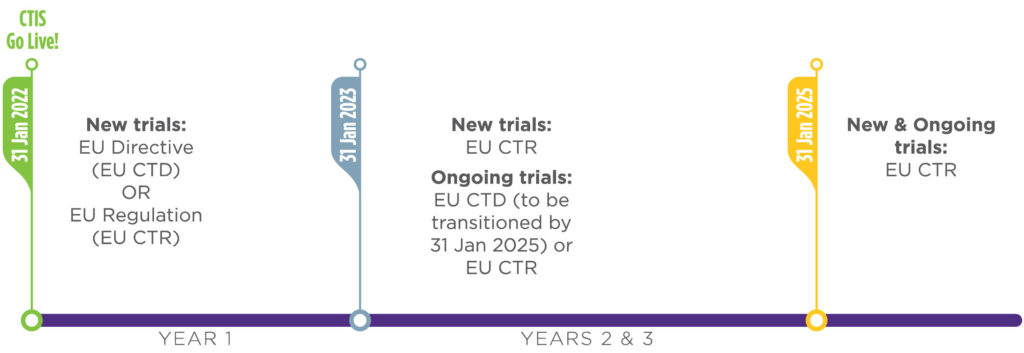
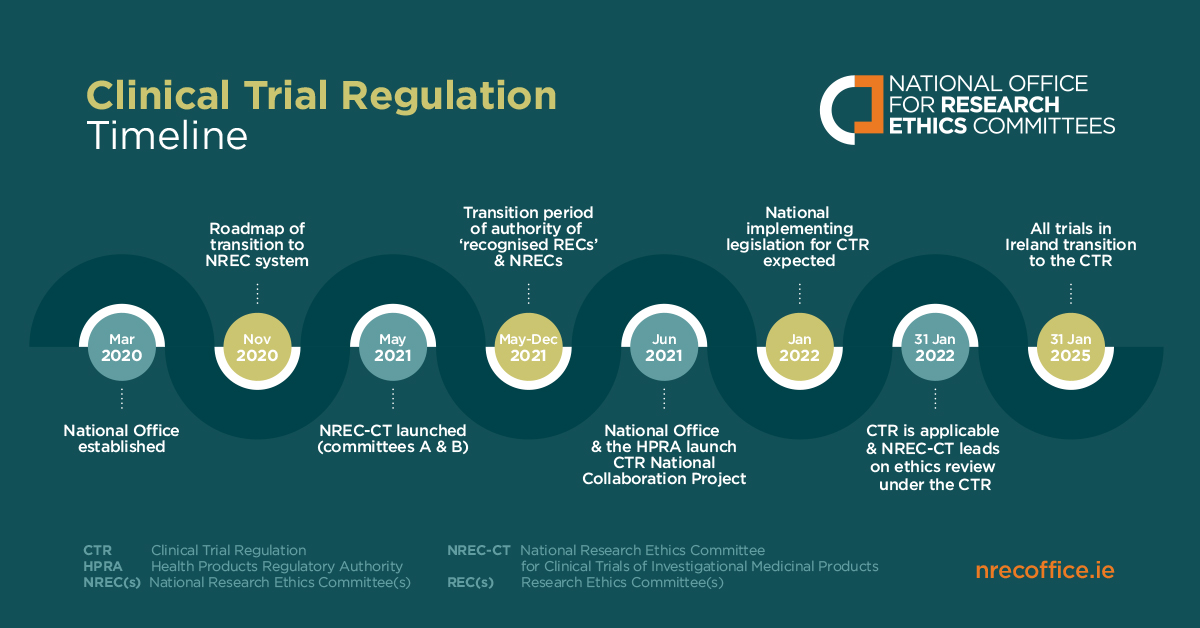
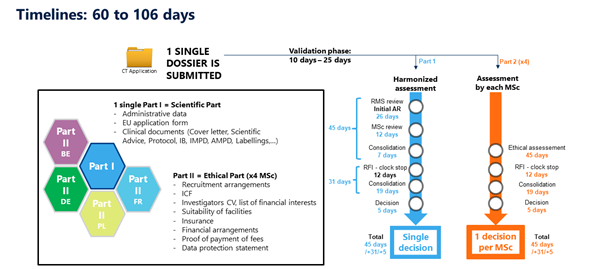
Key considerations to be aware of with Clinical Trial Regulation (CTR) EU No 536/2014 | LINK Medical

MHRA to streamline clinical trial approvals in biggest overhaul of trial regulation in 20 years - GOV.UK

EU Clinical Trial Regulation 536/2014potential timeline. EoT end of... | Download Scientific Diagram

EU Medicines Agency on Twitter: "📢 From today, all initial clinical trial applications in the EU 🇪🇺 must be submitted via #CTIS! All clinical trial sponsors will now use the same system