2022 Executive Certificate Workshop: Regulation of Software as a Medical Device (Conducted Face-to-Face)

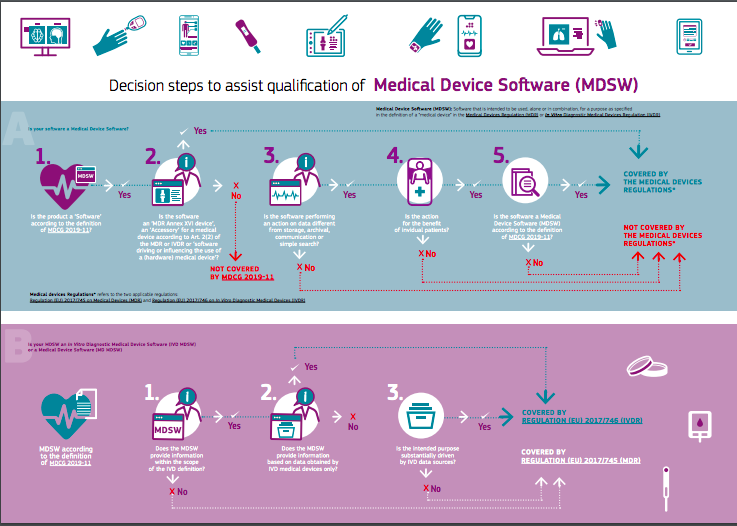
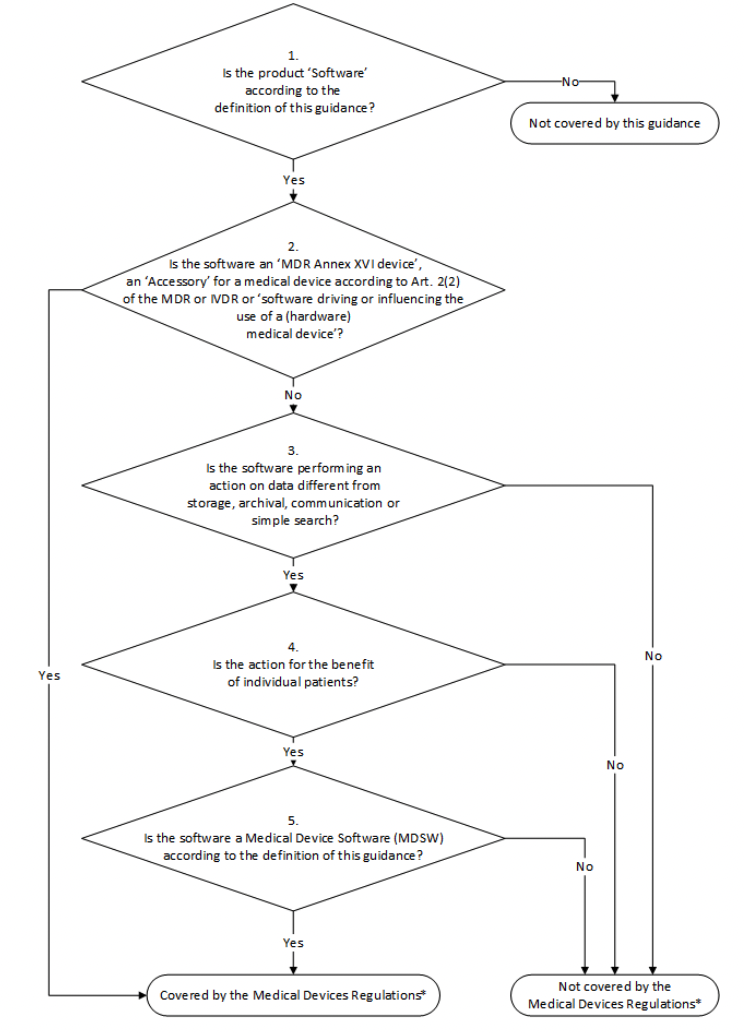
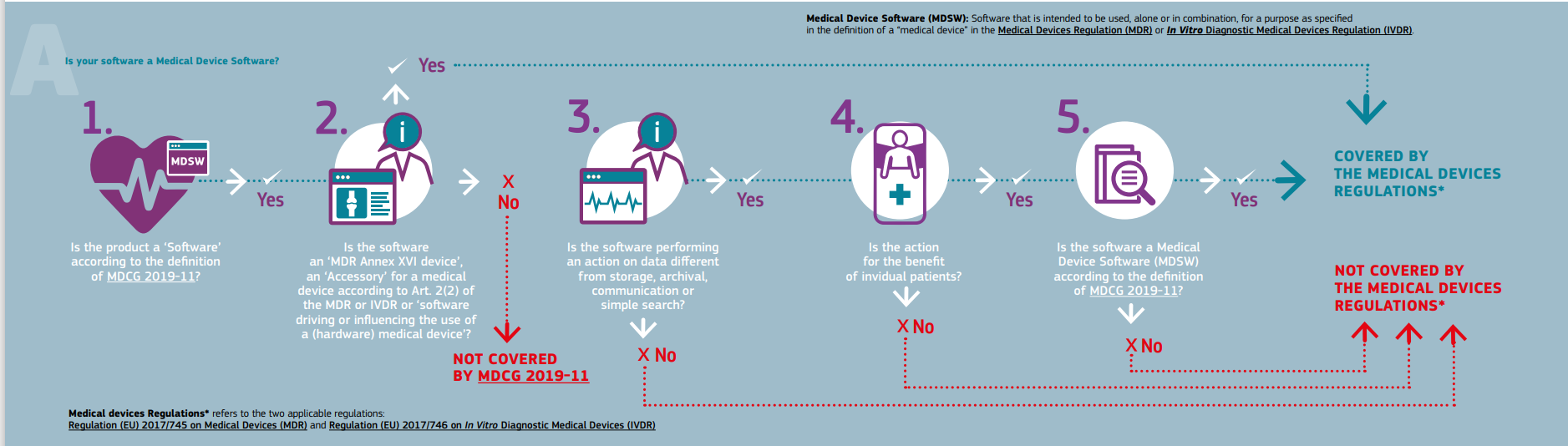
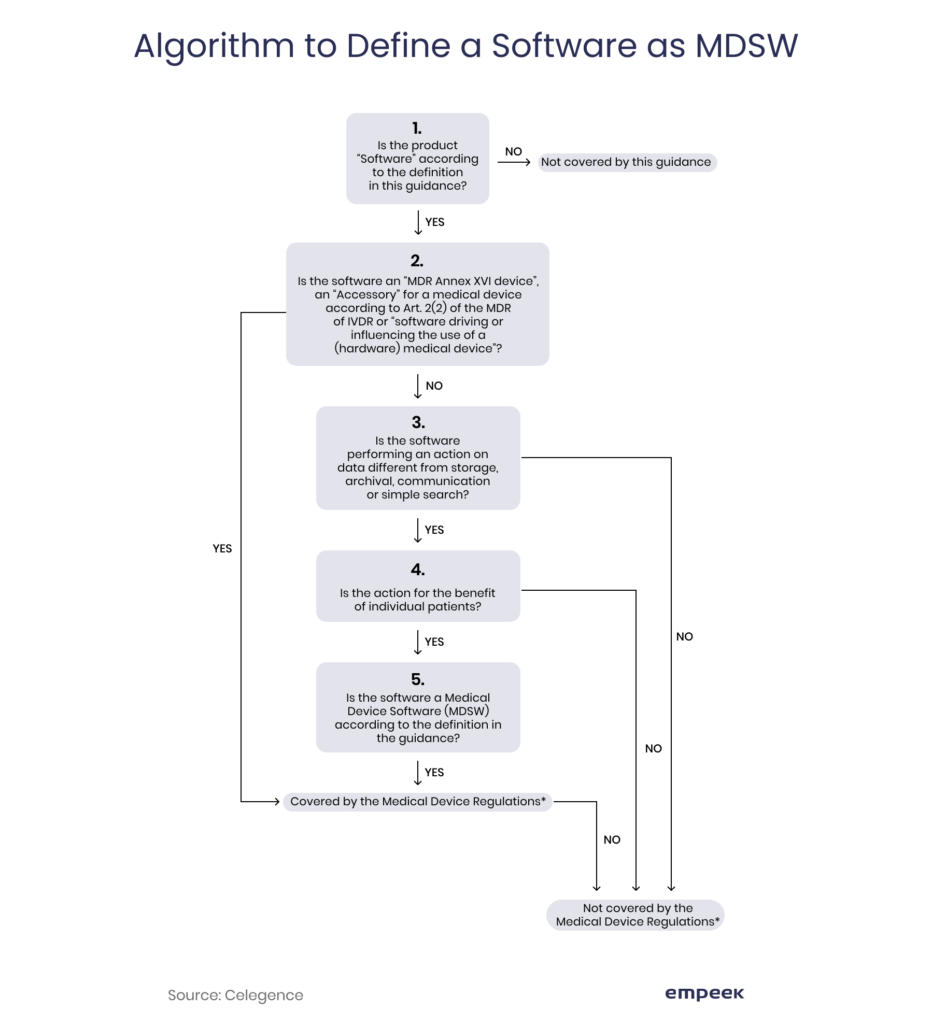
Classification of Software as a Medical device under Medical Device Regulation (European Union) - Kvalito

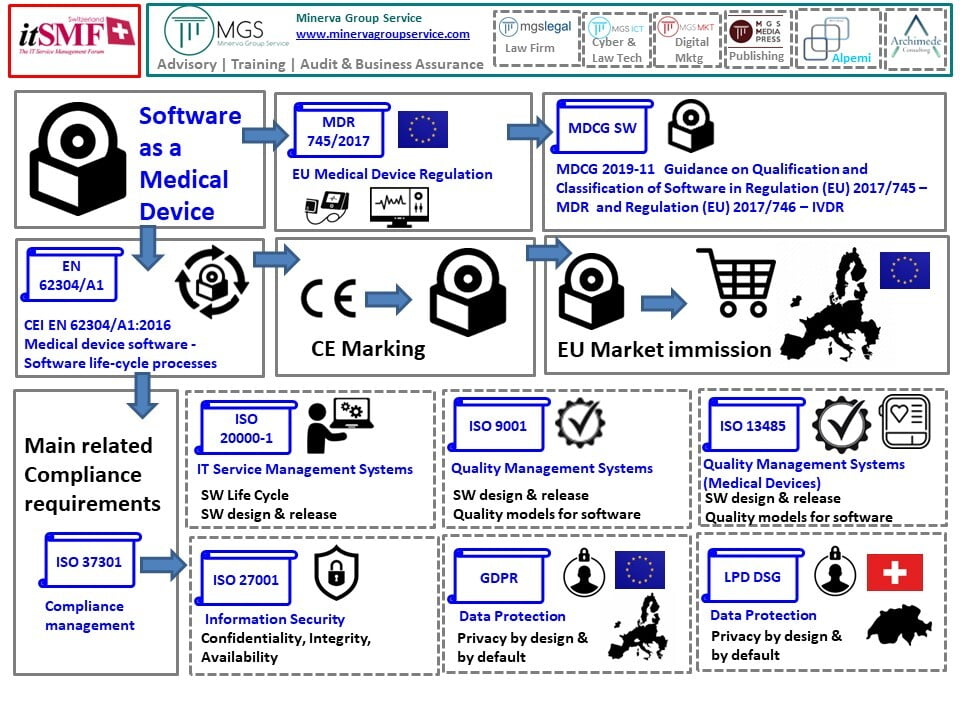
What are the relevant Regulations, Standards, Guidance Documents, Technical Reports after determining my product is a Software as a Medical Device (SaMD)? – Yan Chia

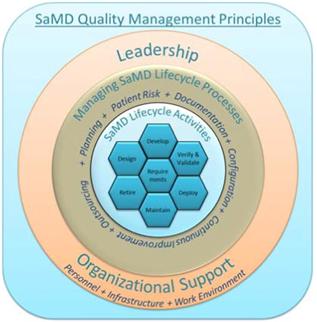
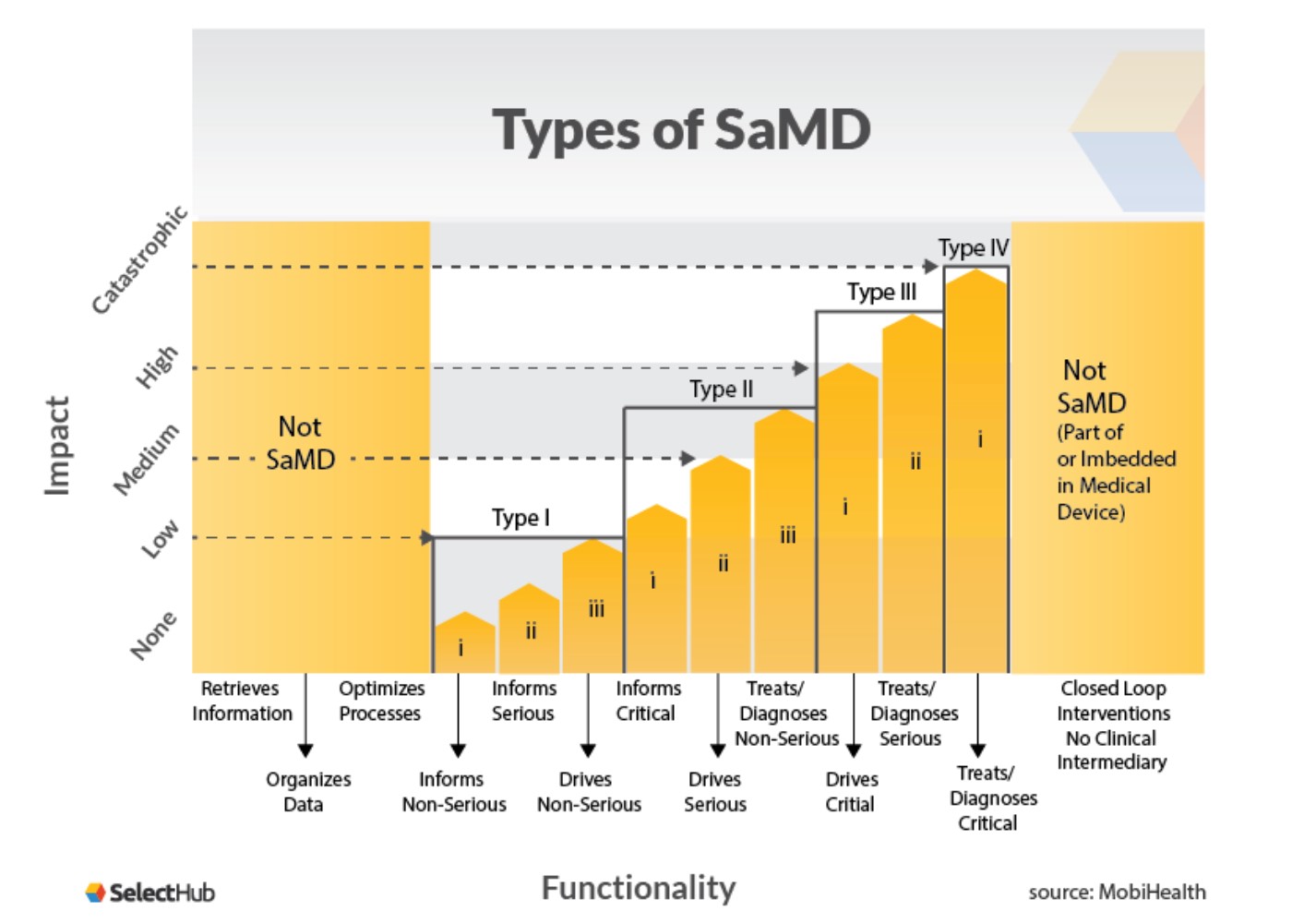
Overview of Regulatory Requirements of Software as Medical Device (SaMD) - Global Regulatory Partners, Inc.
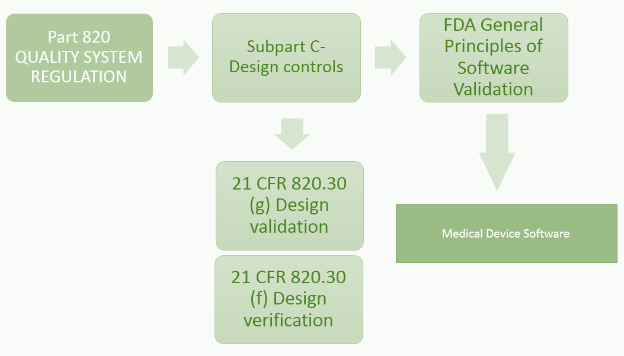
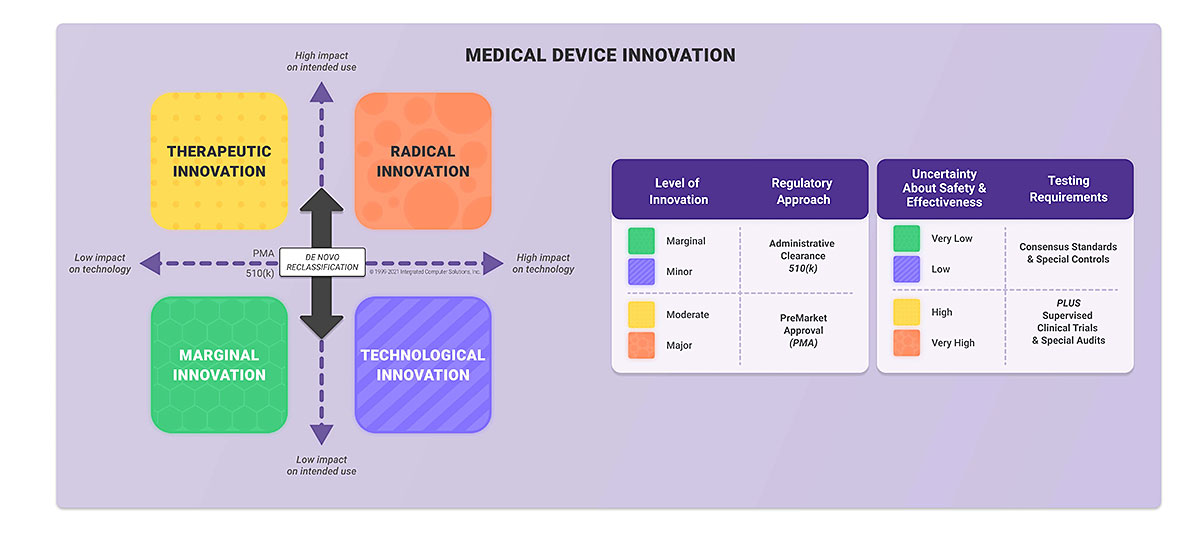
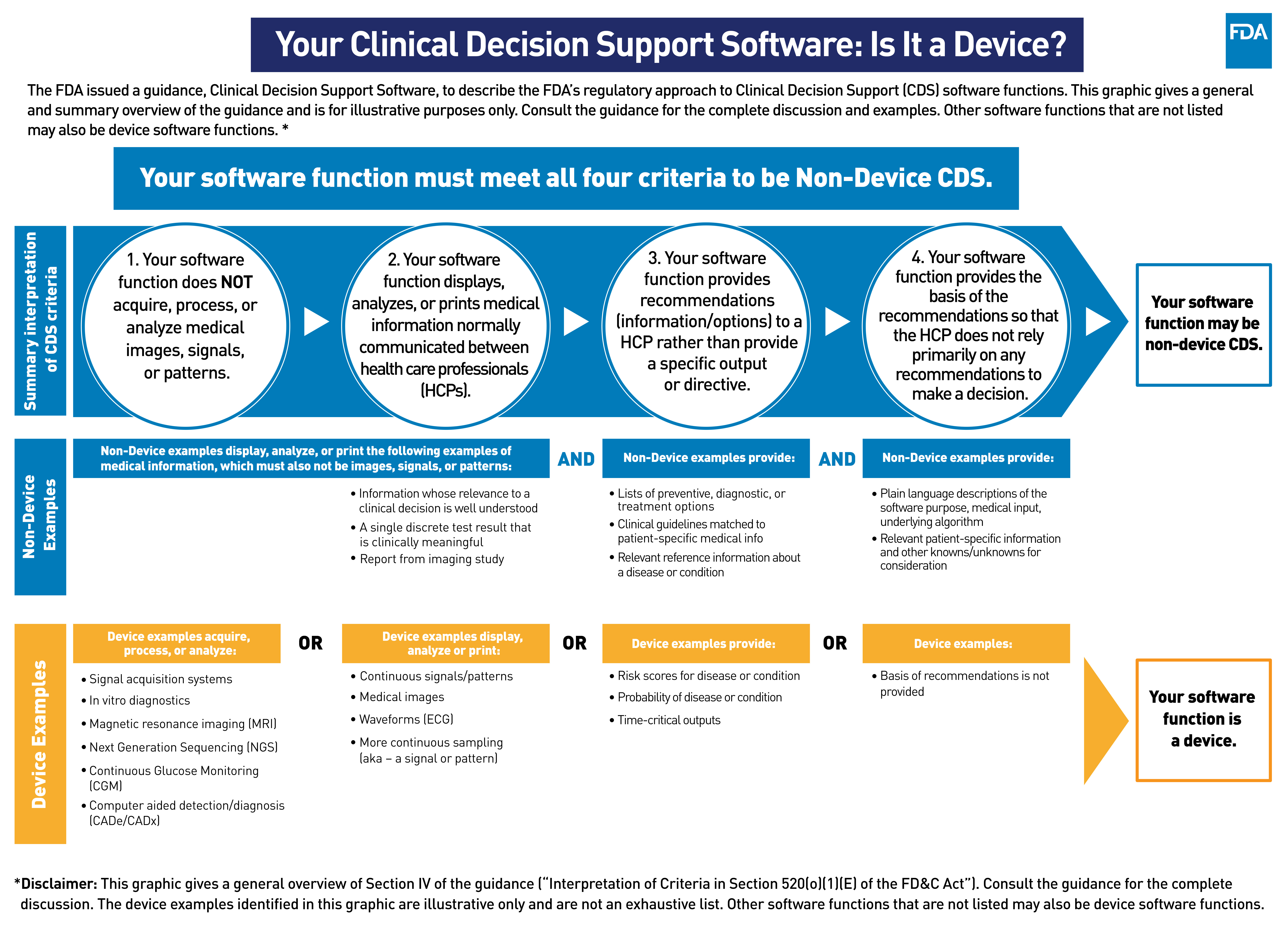
Addressing the Medical Device Software Challenges by understanding FDA's Software Regulation Strategy