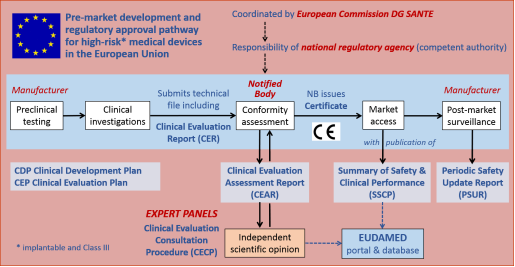
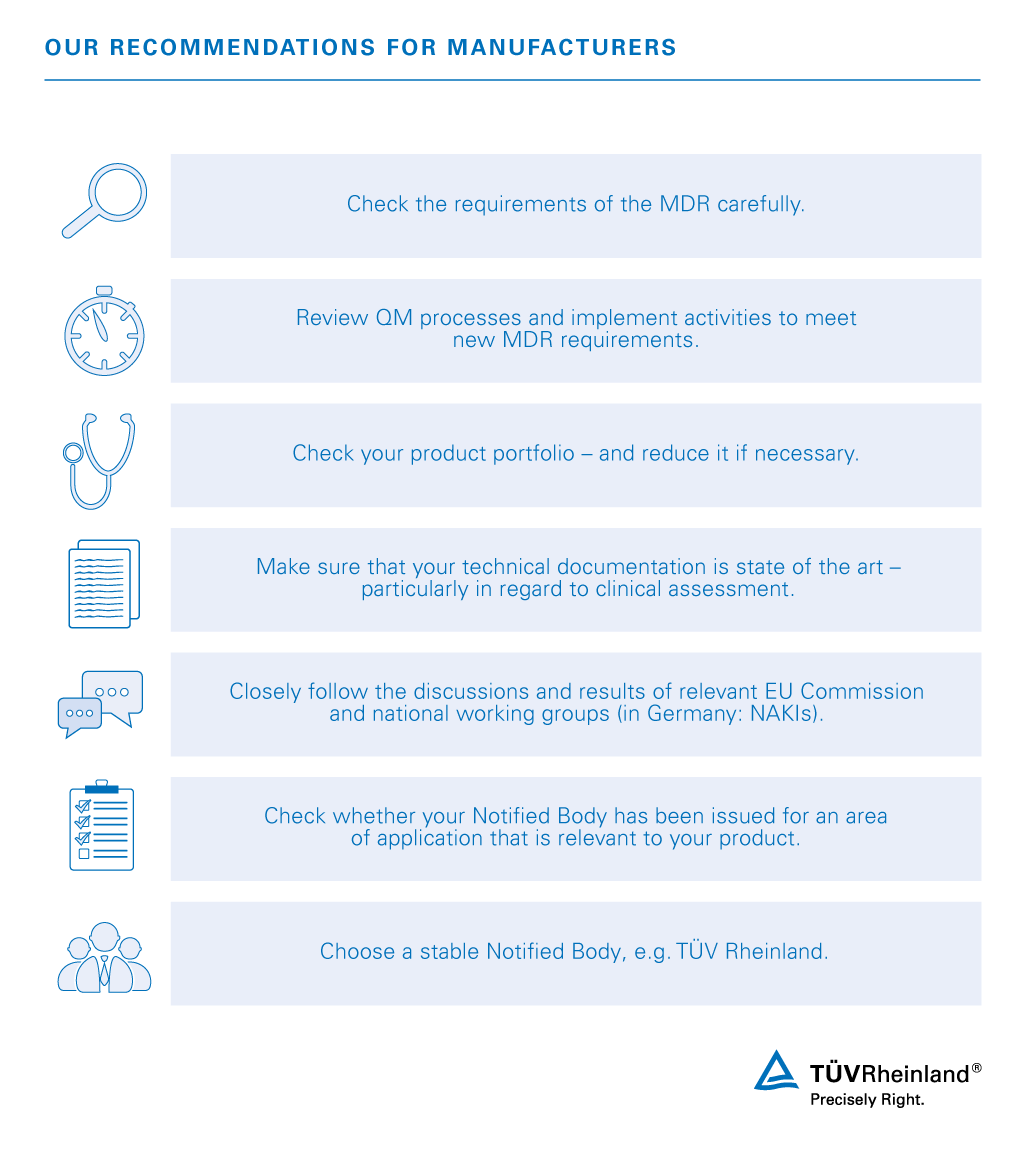
European Medical Device Regulation (MDR) for MedTech and Medical Device Manufacturers : O'Brien, Mr Des: Amazon.es: Libros

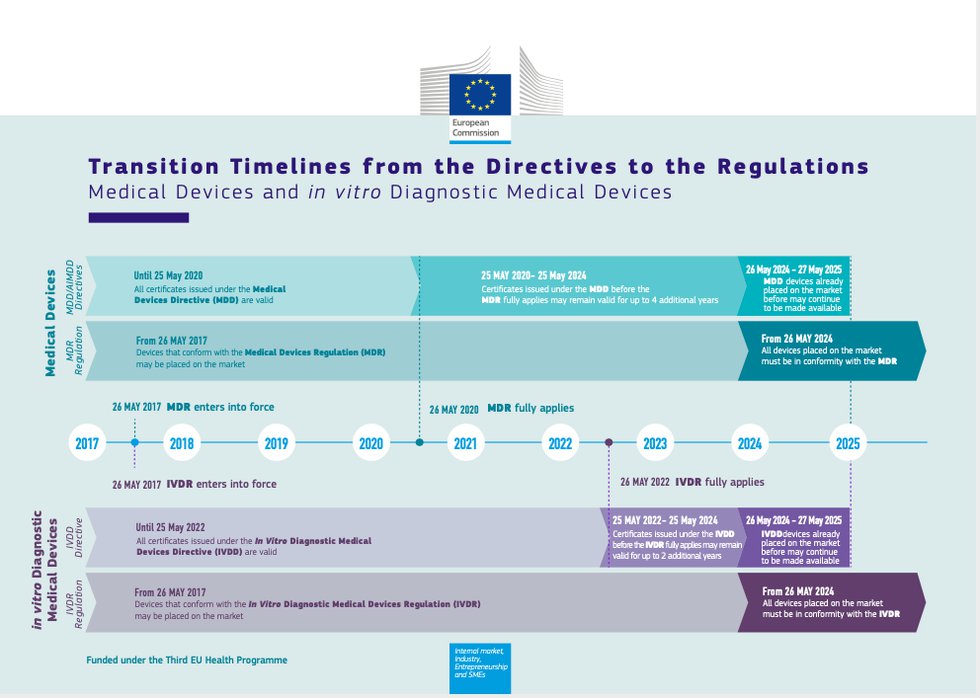
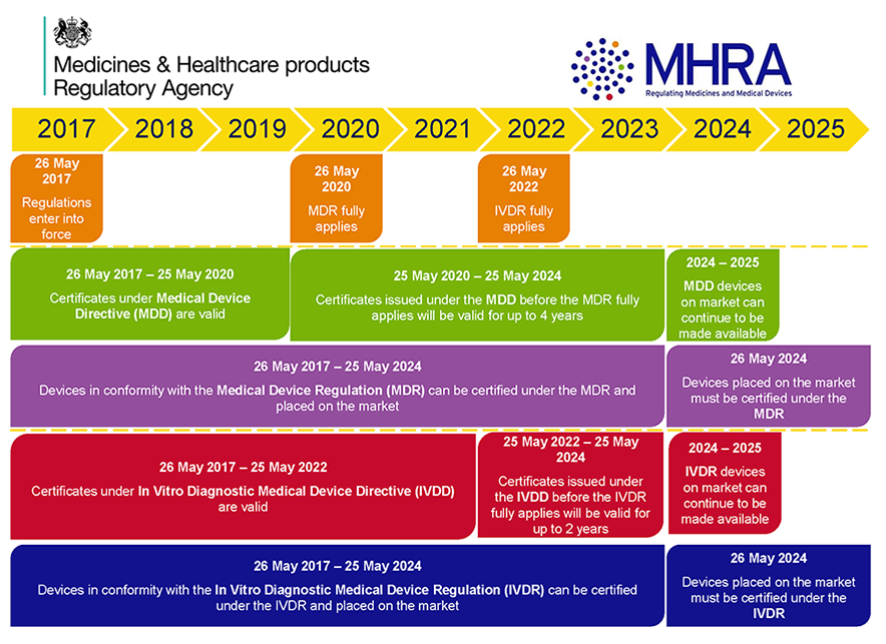
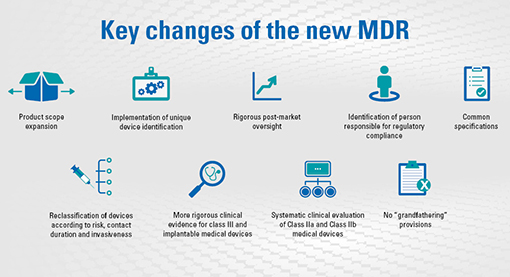
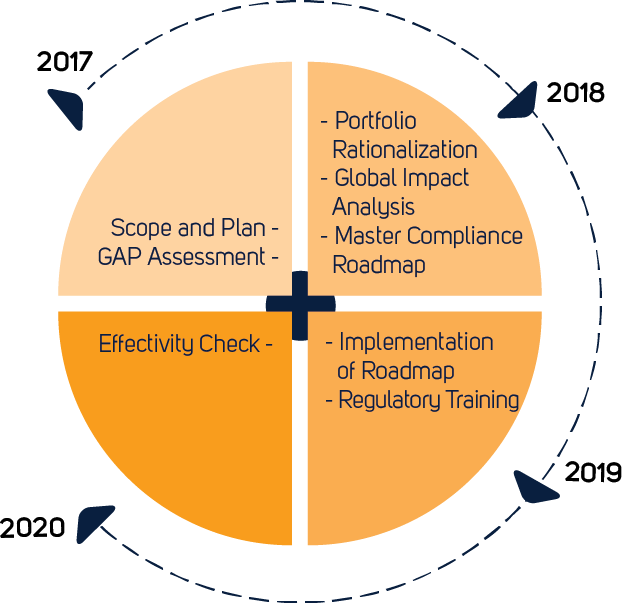
EU Finalizes New Medical Device Regulations (MDR) which update the regulatory framework for the marketing of devices and IVDs in Europe – Catchtrial