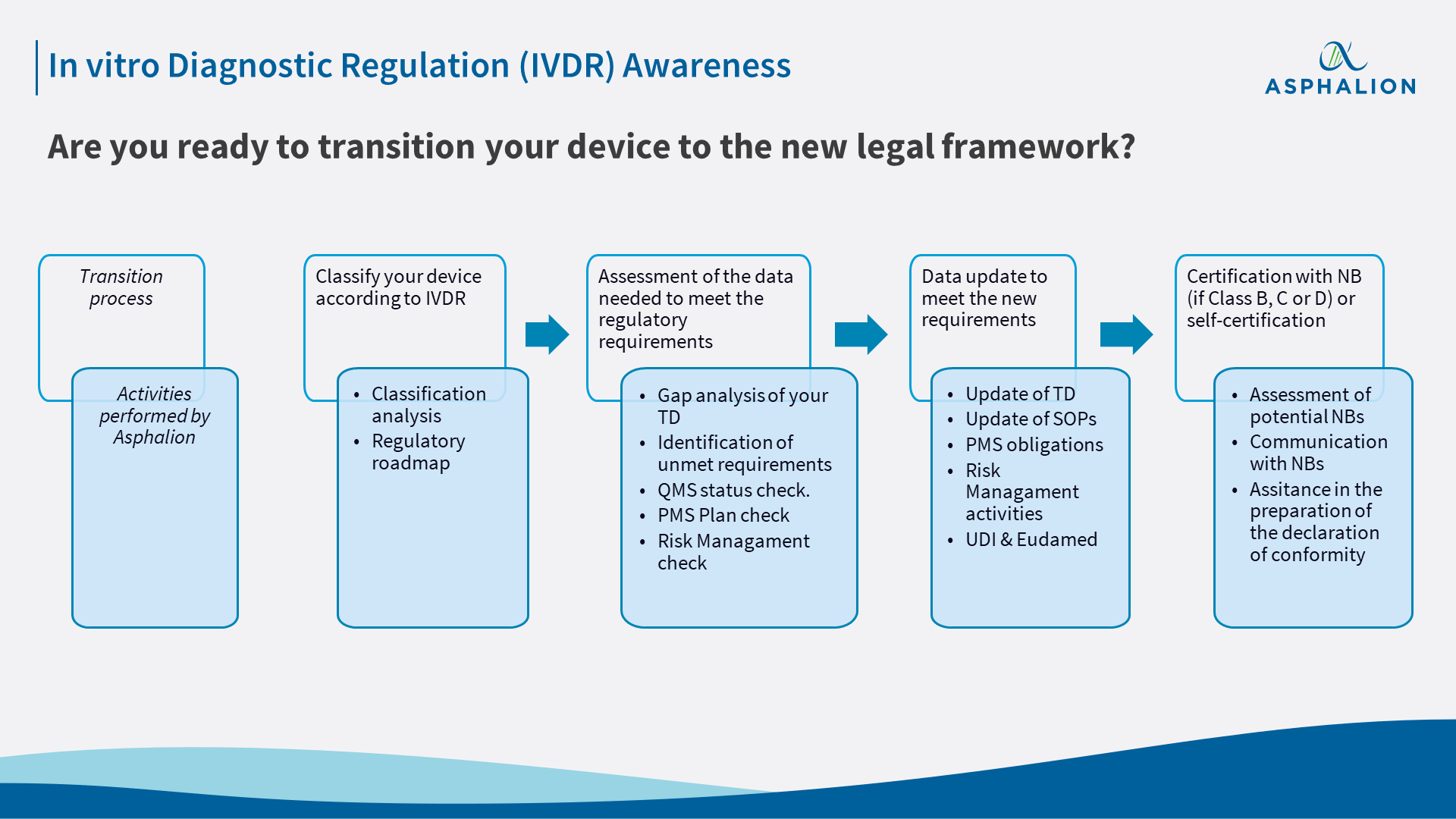
Preparation is key: brief checklist how to bring IVD MD into compliance with EU IVDR - Biotech Spain

The role of m6A-autophagy axis in the regulation of IVDD development... | Download Scientific Diagram

Chondrogenic regulation of PDB therapy in DM-induced IVDD mice. (A)... | Download Scientific Diagram



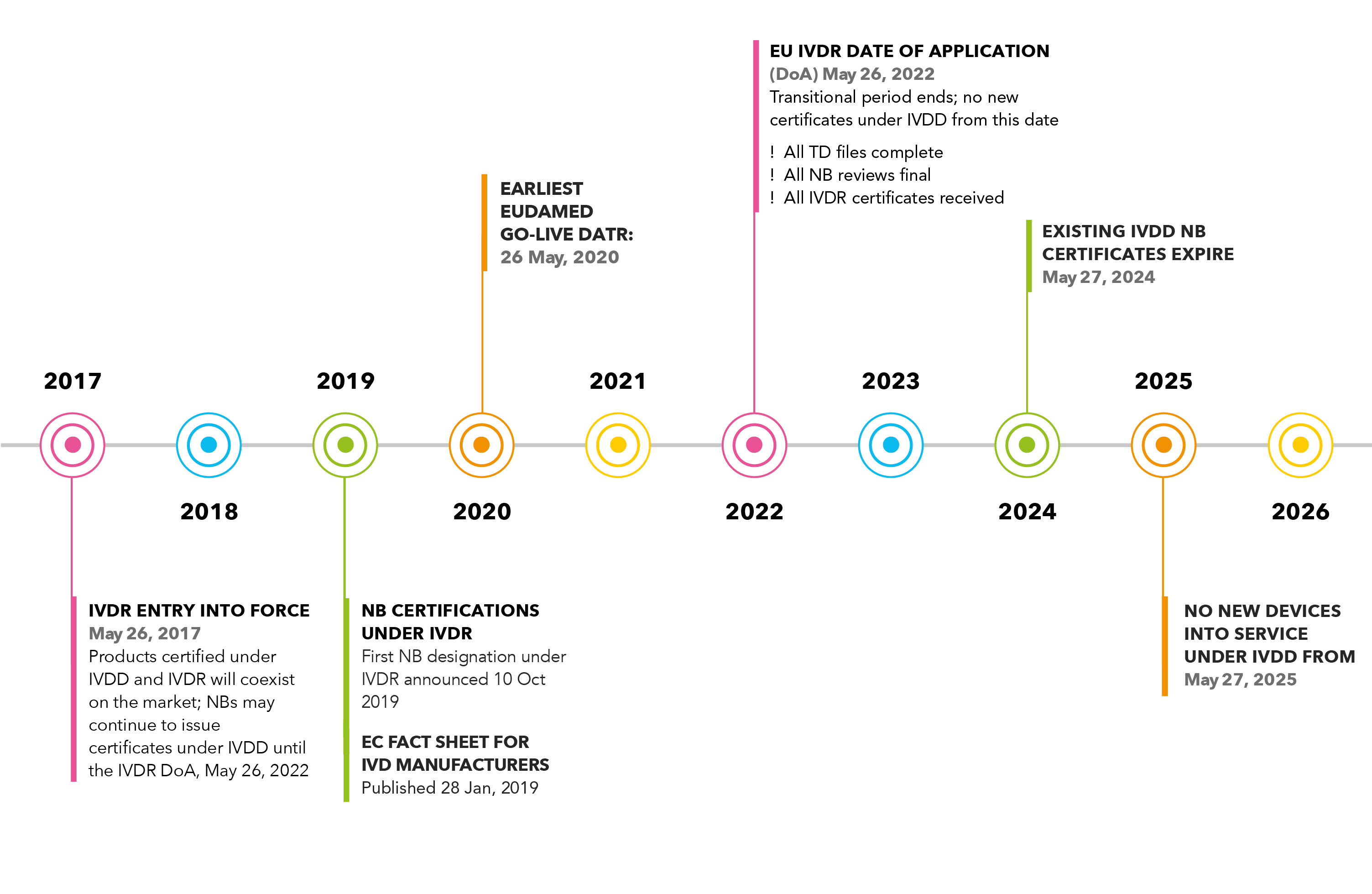

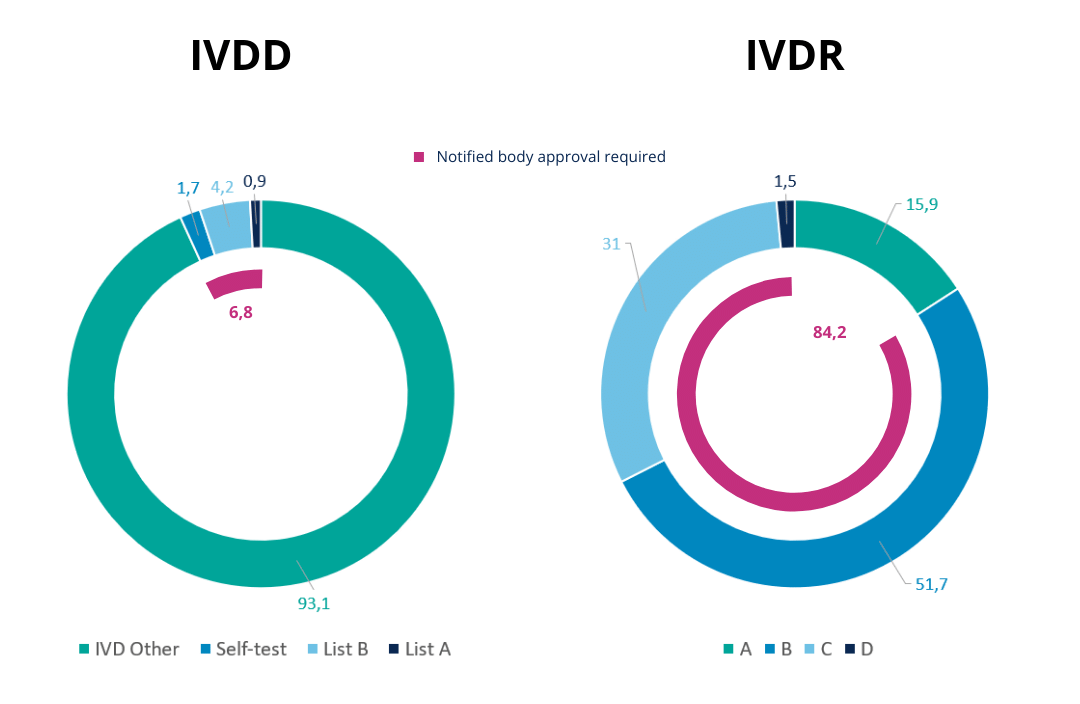
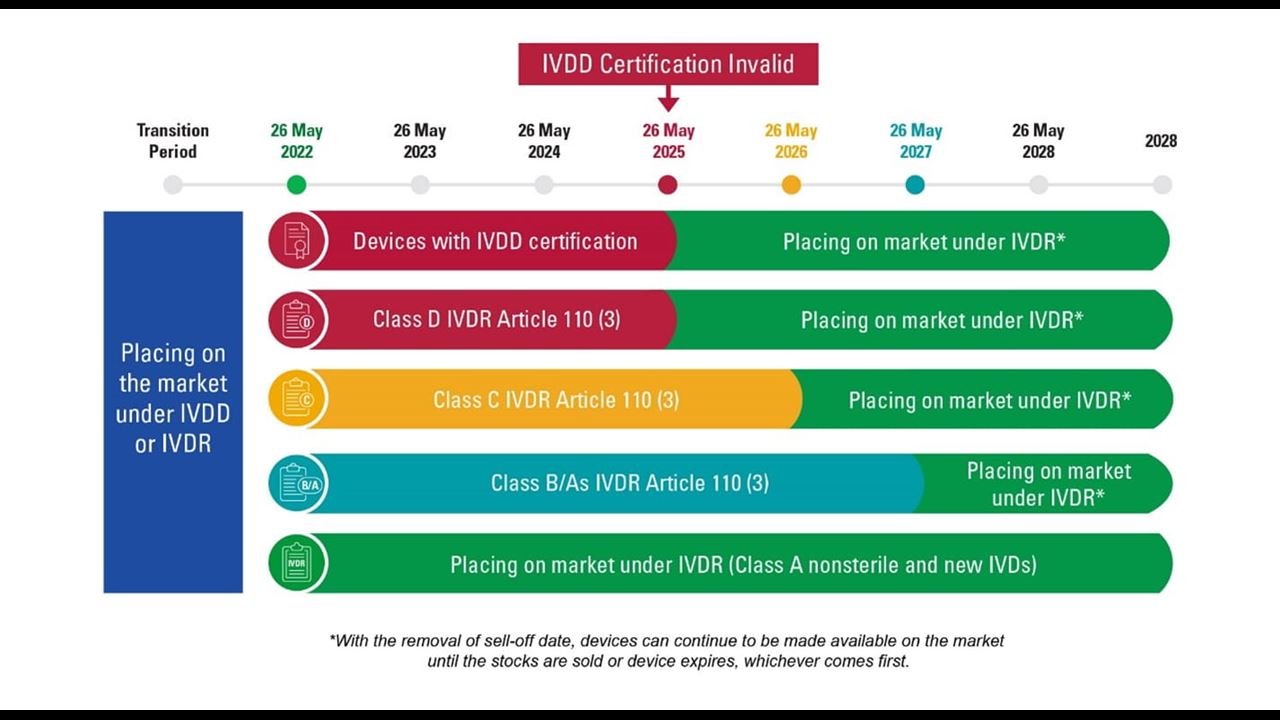


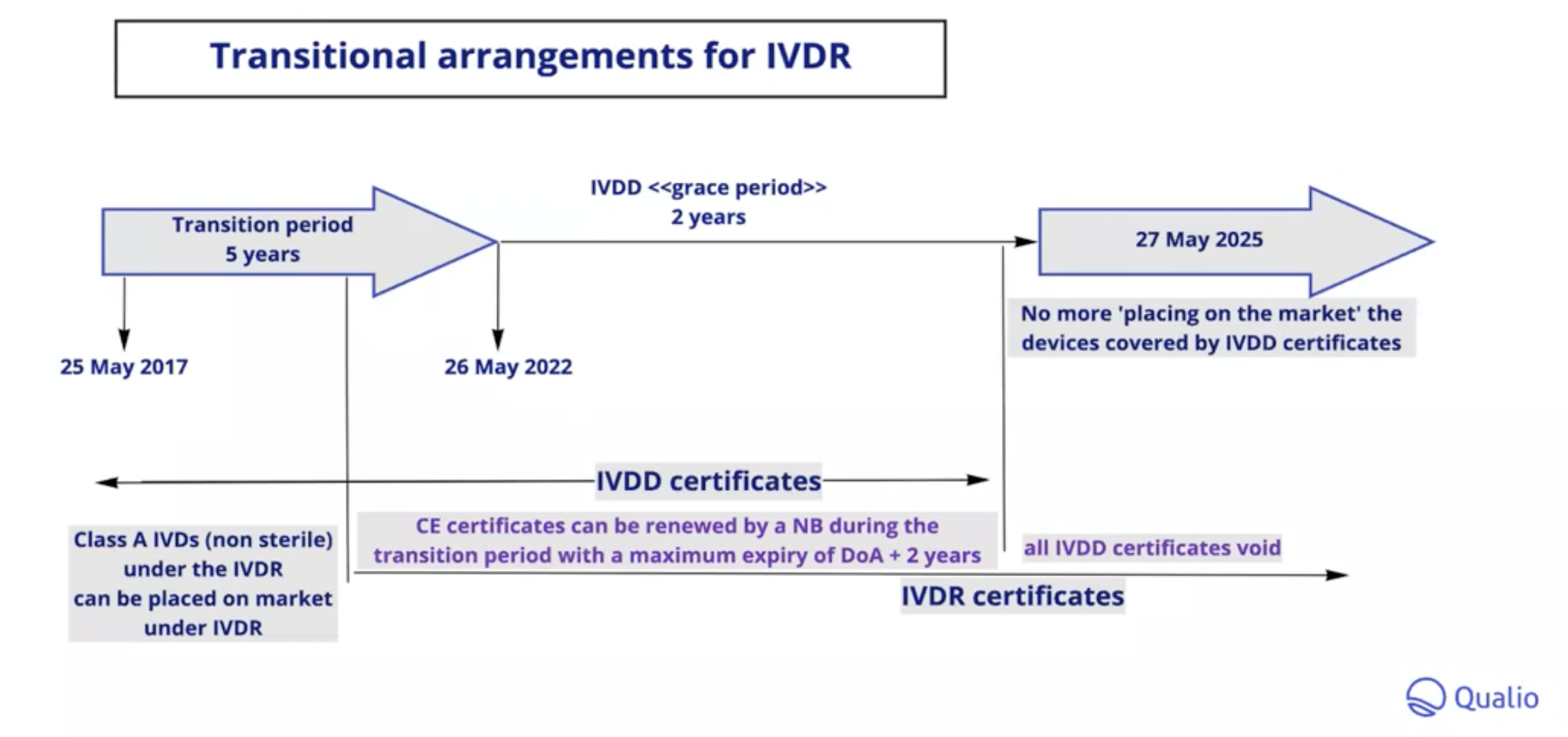
/tuv-rheinland-ivdr-visual-2-en_core_1_x.png)
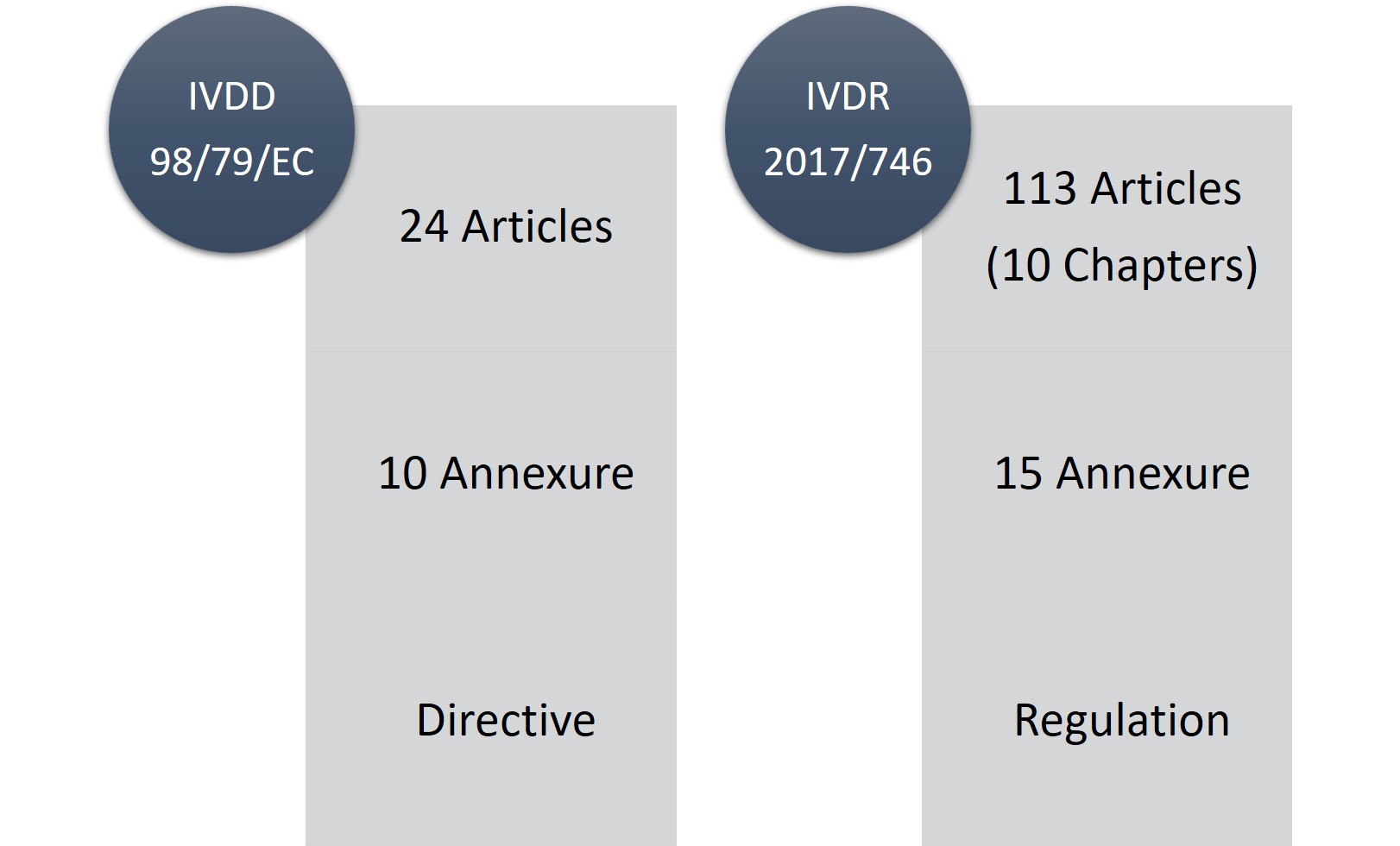


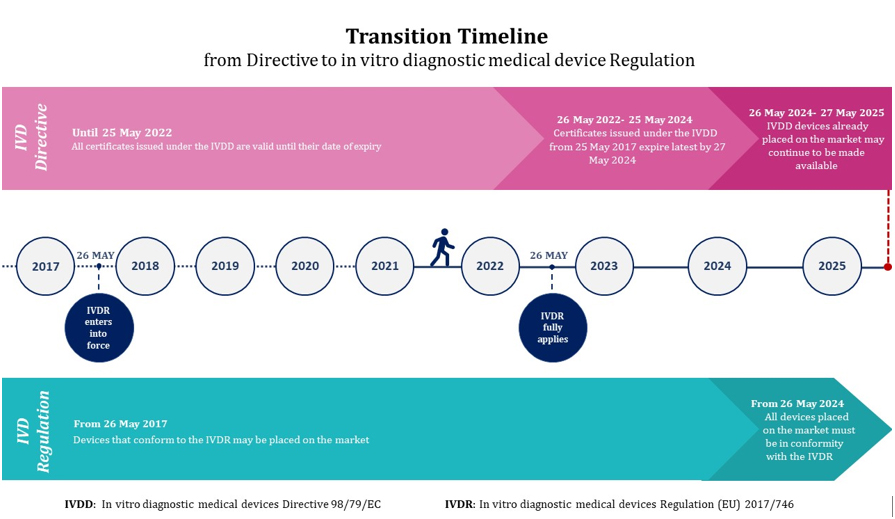
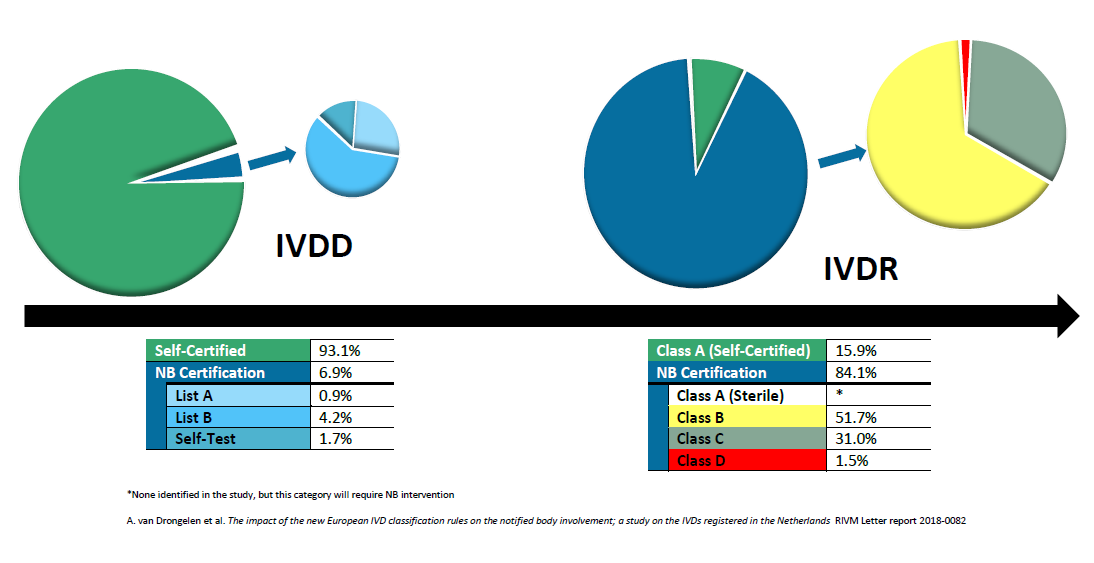

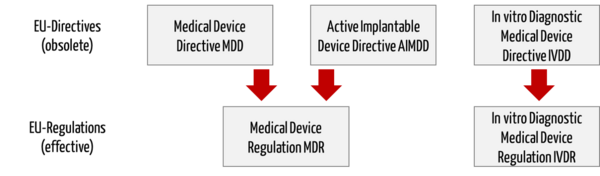
/tuv-rheinland-ivdr-visual-1-en_core_1_x.png)