Medical device regulation in Europe – what is changing and how can I become more involved? - EuroIntervention

Stella Kyriakides on Twitter: "📍In vitro medical devices protect and save lives! 👉By rolling out the new In Vitro Medical Devices Regulation progressively, we can ensure that these essential products continue to

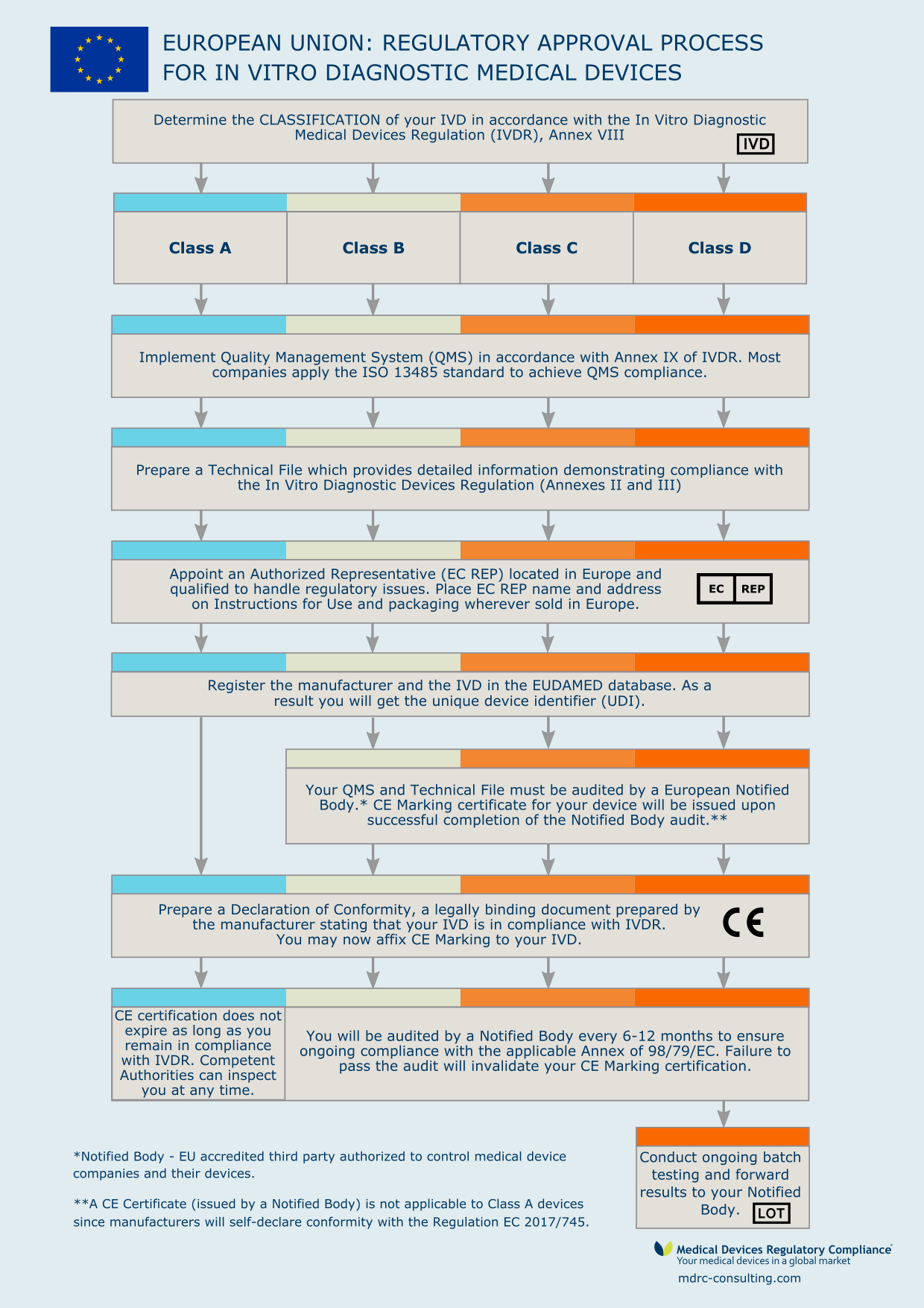
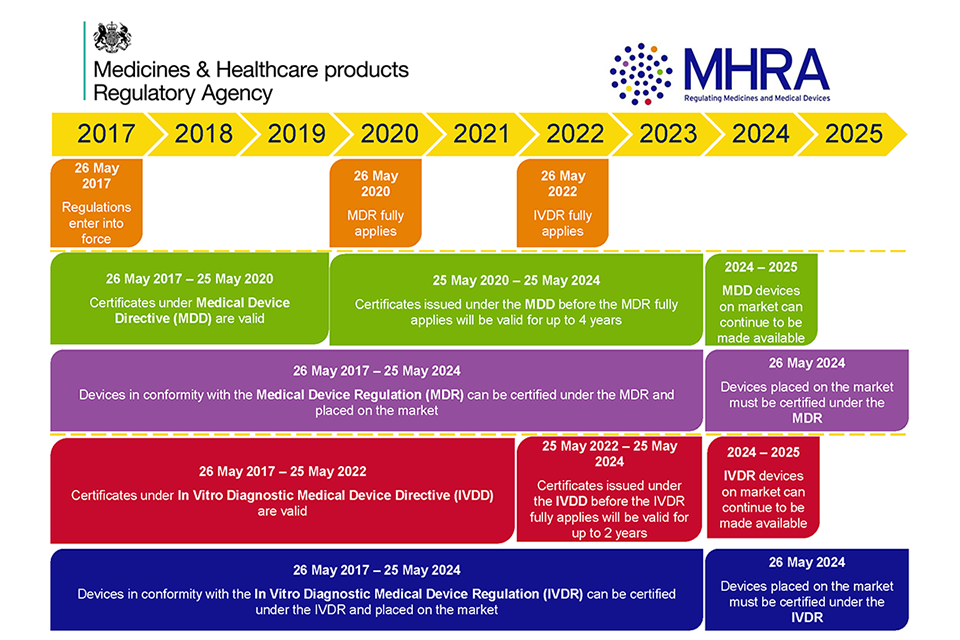
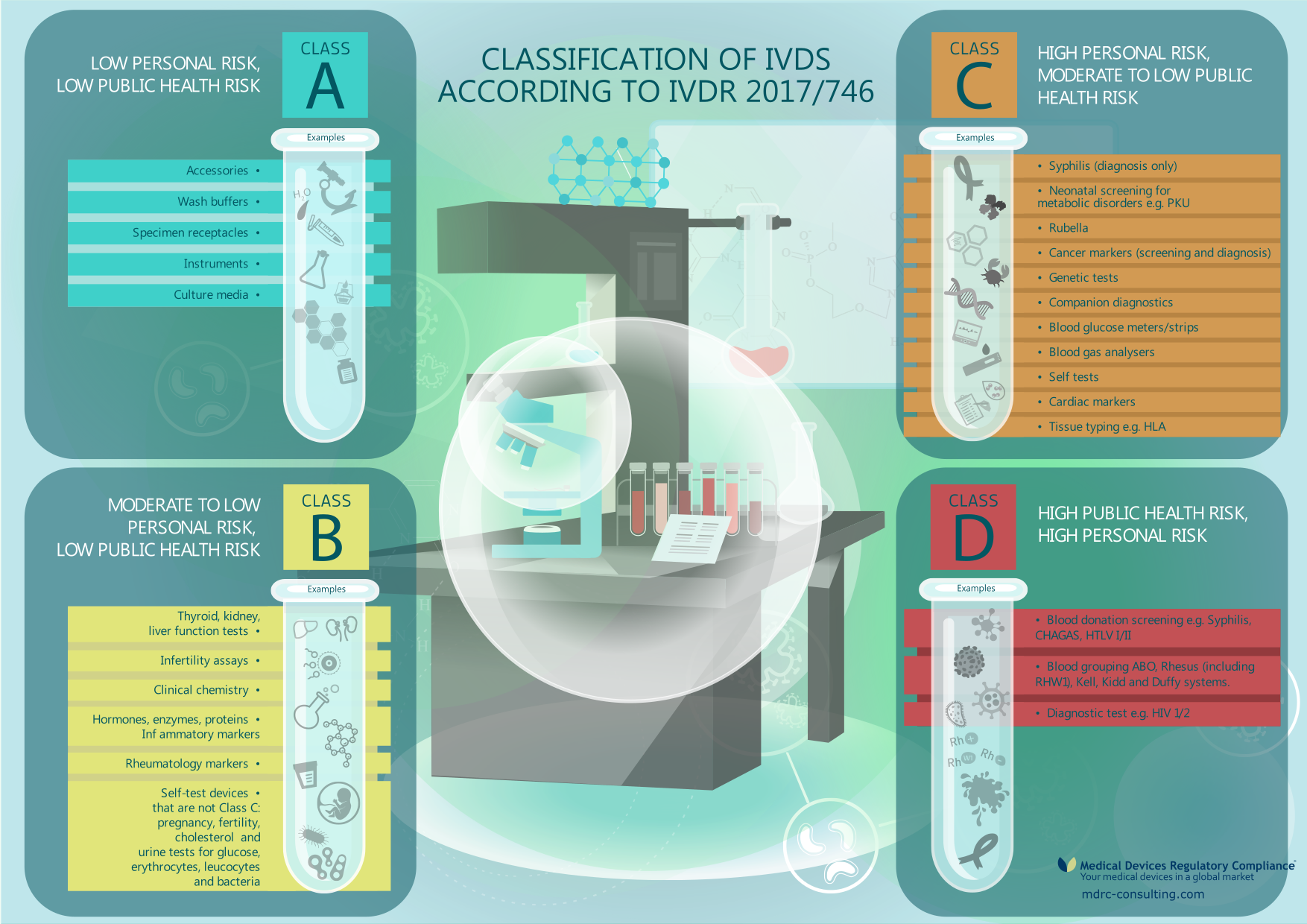
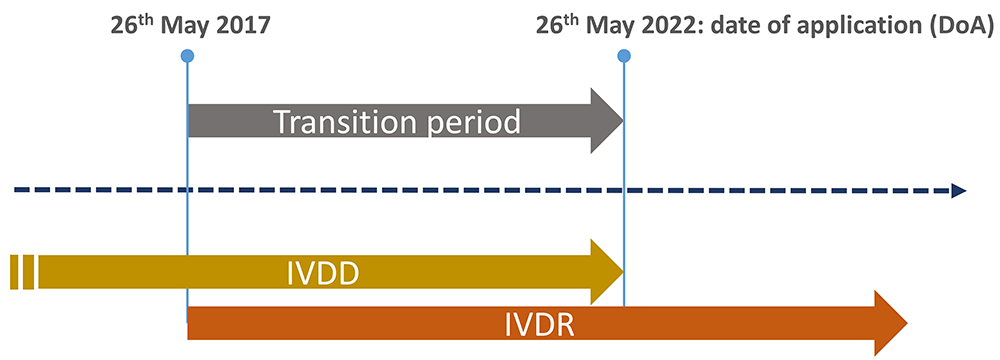
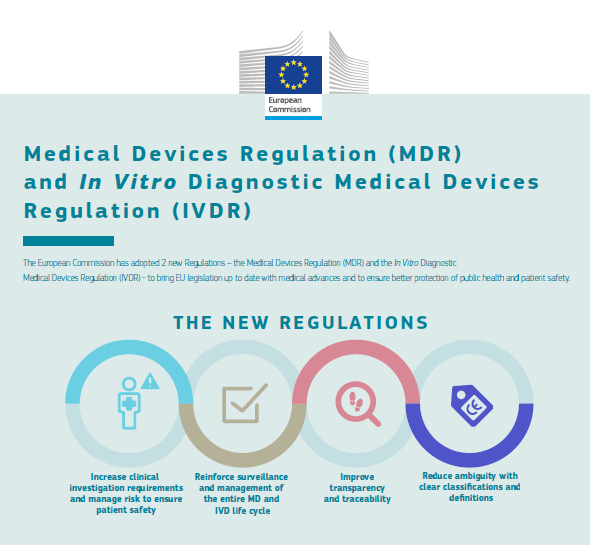
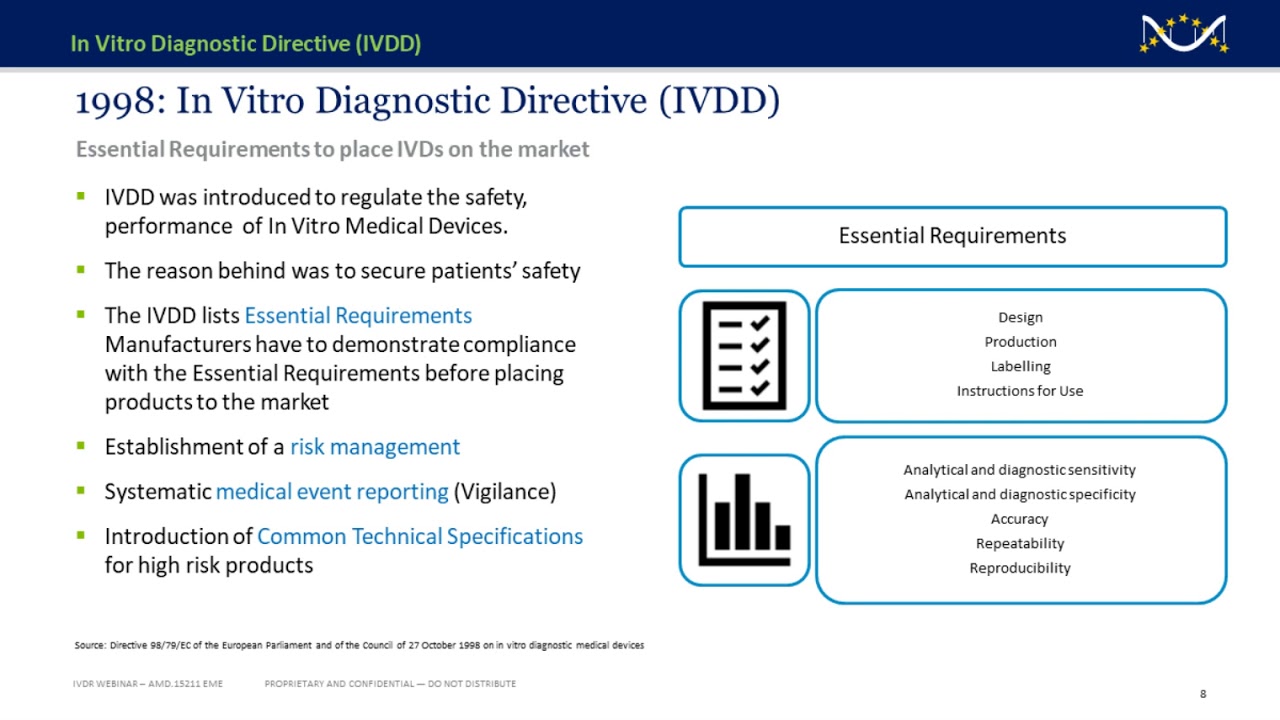
The In vitro diagnostic medical devices regulation (EU) 2017/746: what will change for public health

Publication of the European Regulation 607/2023 on Medical Devices and In Vitro Diagnostic Medical Devices. - Burotec Sanitary Product






/tuv-rheinland-ivdr-visual-2-en_core_1_x.png)