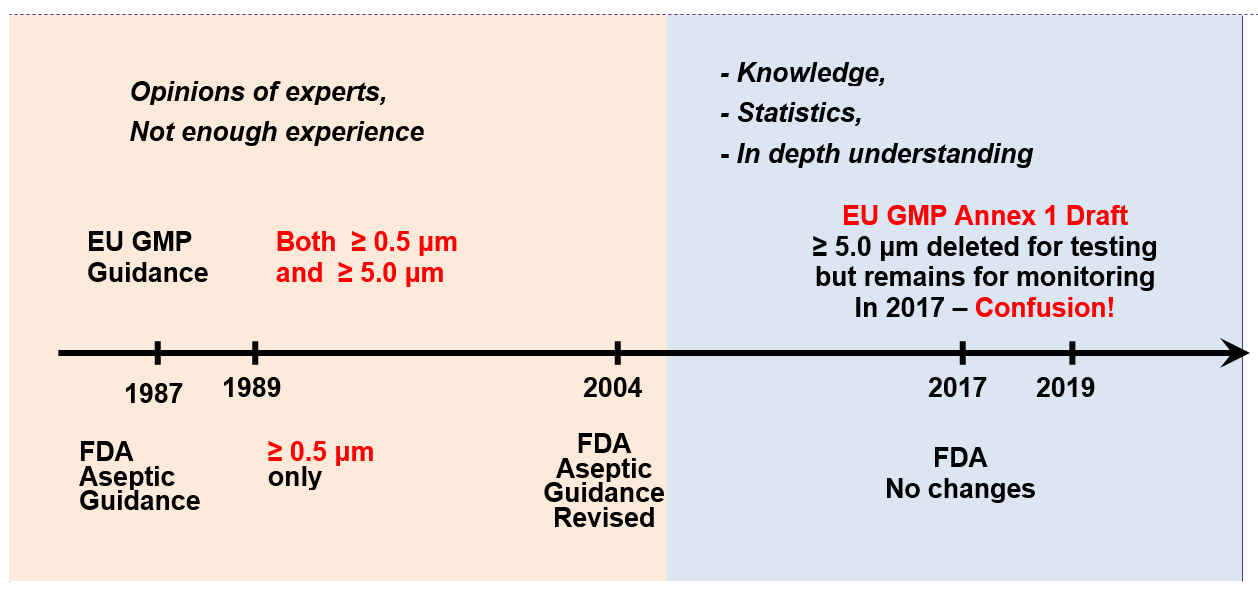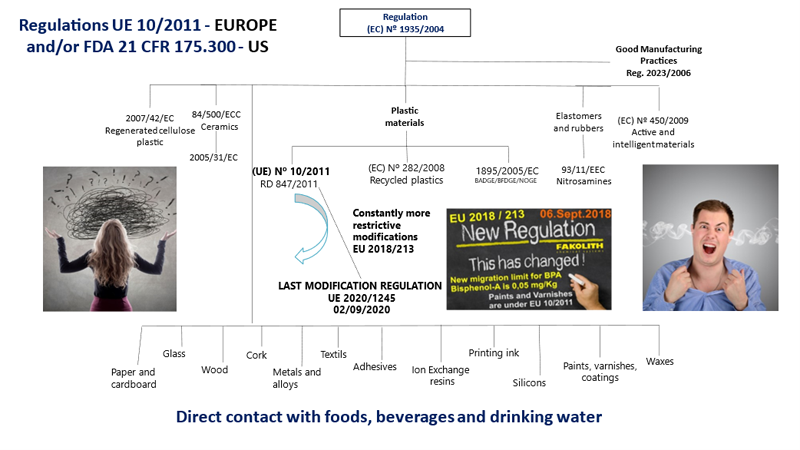
Declaration of Conformity/Compliance - Food Contact Materials and Coatings - EU 10/2011 and FDA 21 CFR 175.300 - FAKOLITH
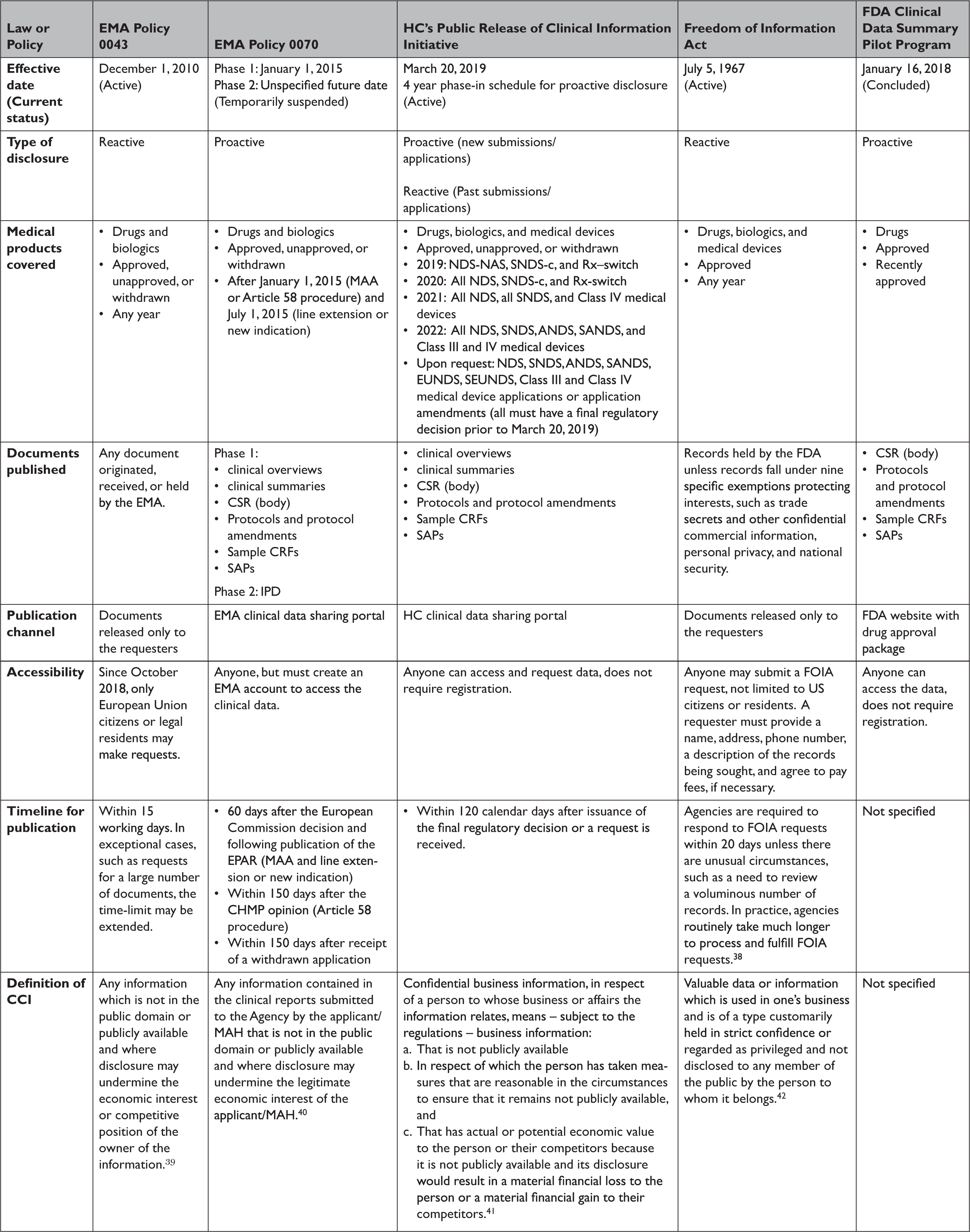
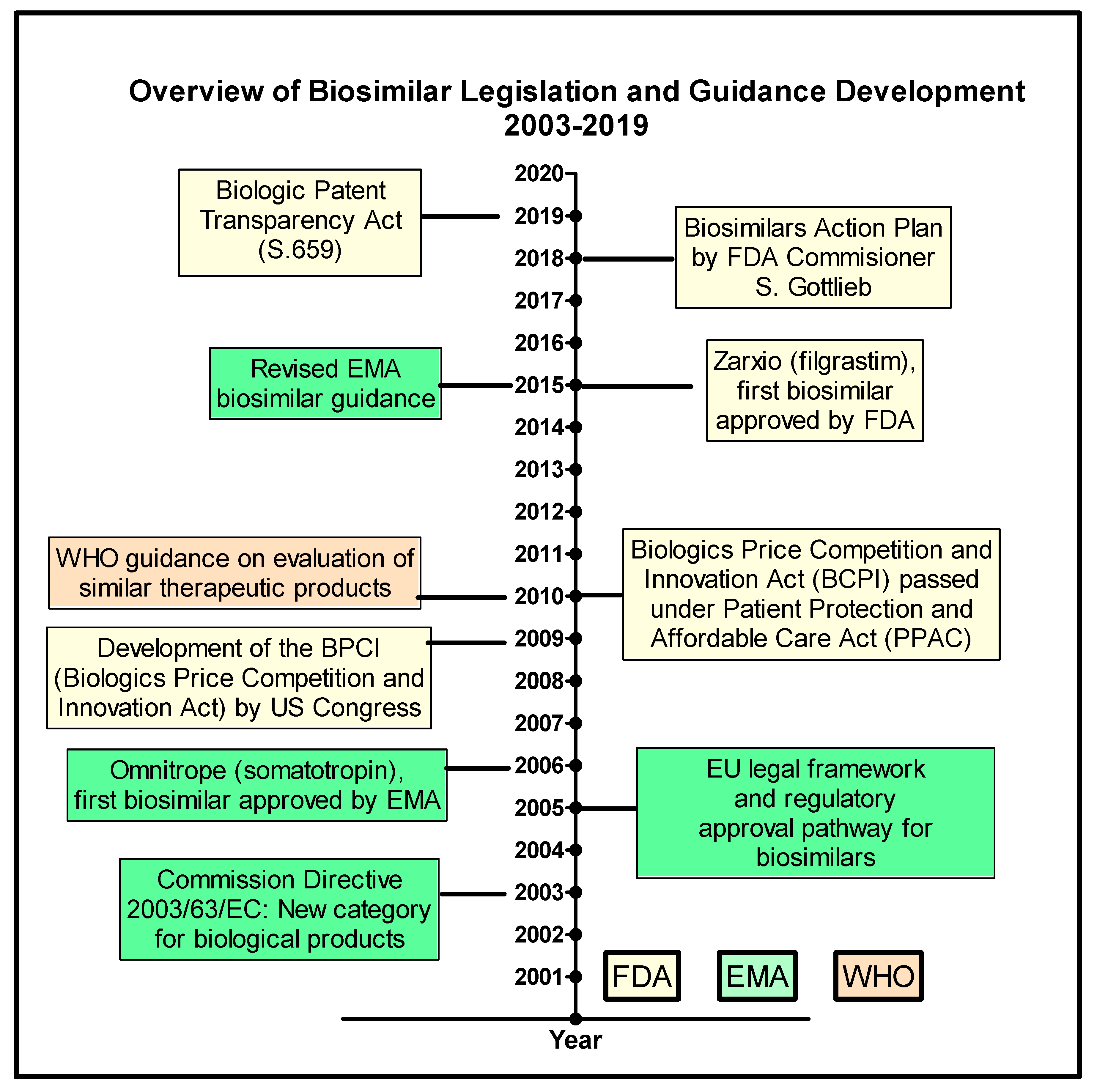
Transparency of Regulatory Data across the European Medicines Agency, Health Canada, and US Food and Drug Administration | Journal of Law, Medicine & Ethics | Cambridge Core
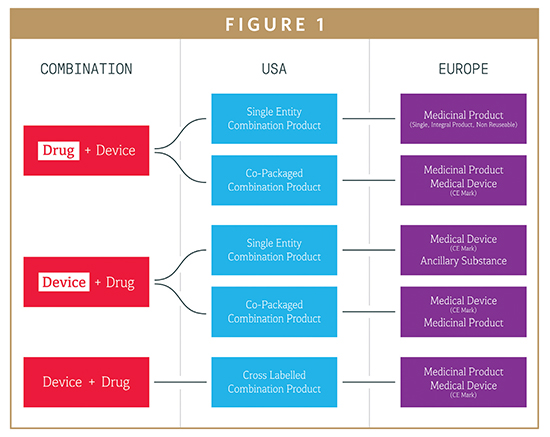
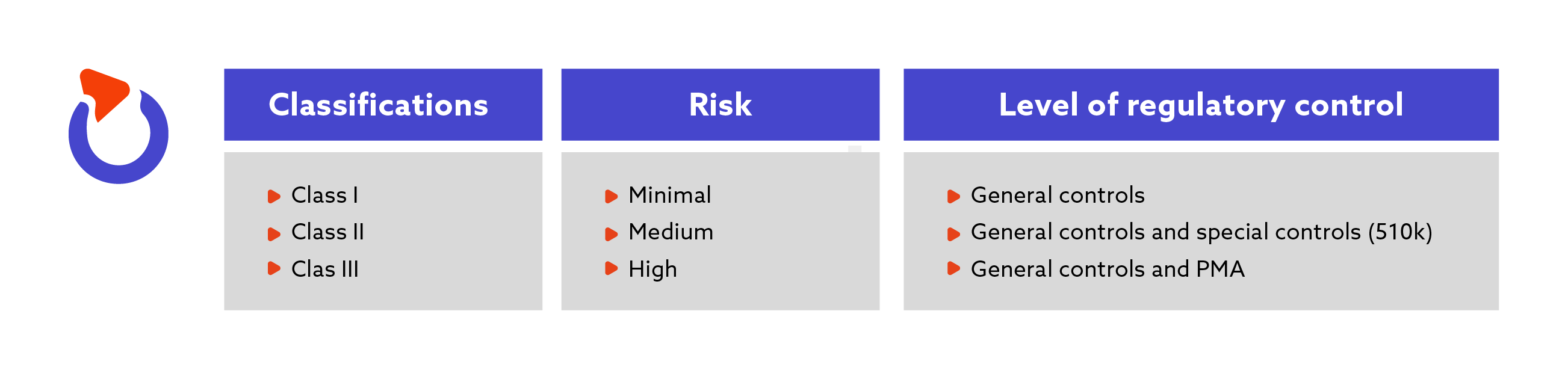
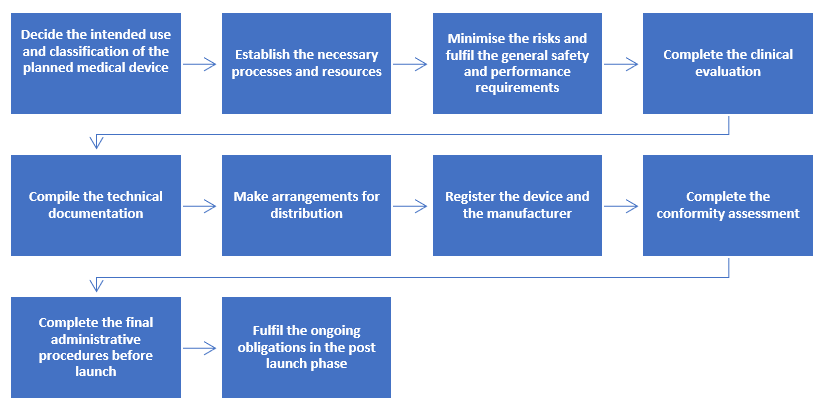
DEVICE REGULATIONS - The New Medical Device Regulation & the Applicability of Article 117 to Medicinal Products

Approval of artificial intelligence and machine learning-based medical devices in the USA and Europe (2015–20): a comparative analysis - The Lancet Digital Health