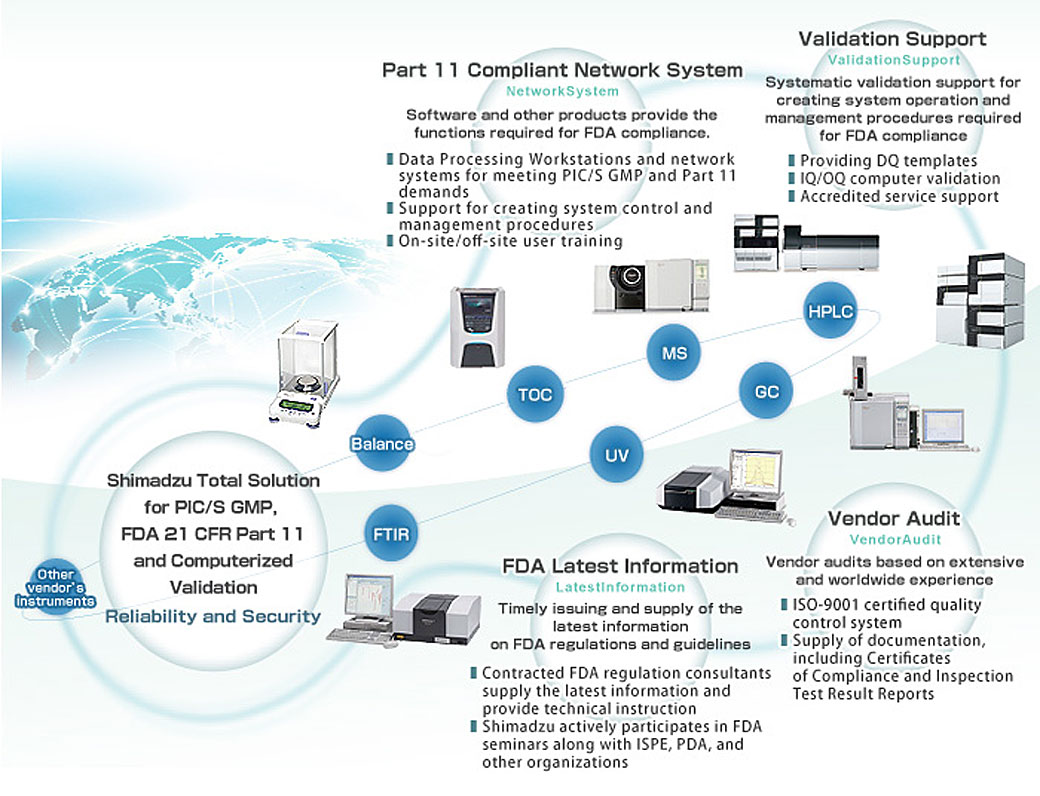
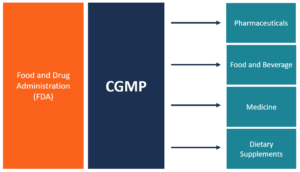
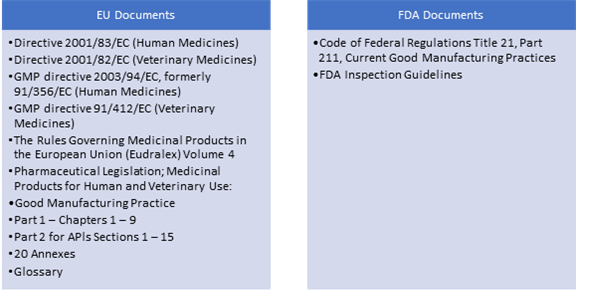
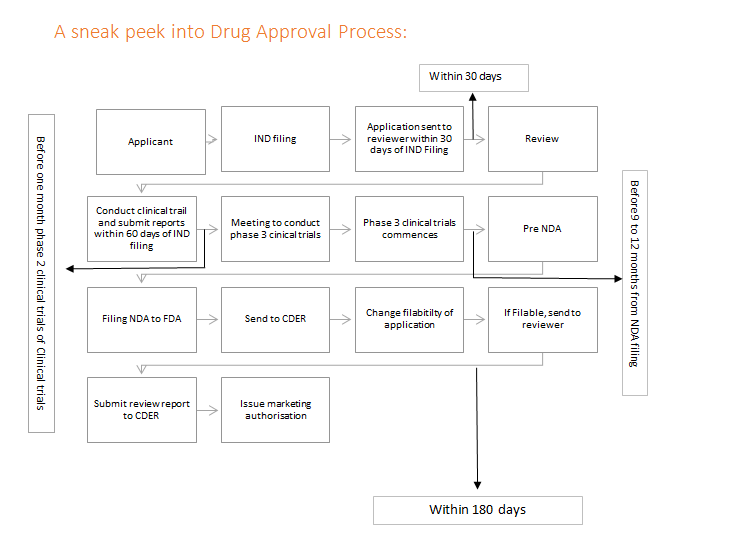
US FDA, EU GMPs, ICH Guideline, Japanese GMPs Handbook (Code of Federal Regulations) - Food And Drug Administration: 9781935131304 - IberLibro
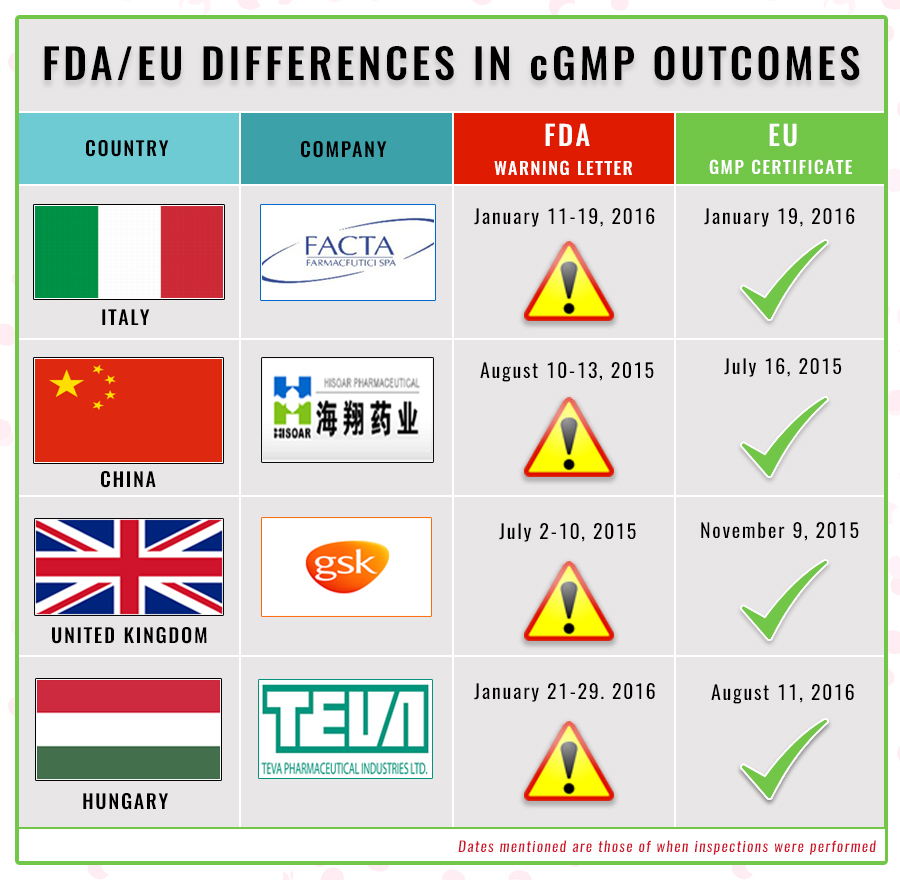
FDA and EU Differ on cGMP Standards at the Same Facilities: How will they Mutually Recognize Inspections? | Radio Compass Blog

![GMP Compliance of Foreign Suppliers: The Importers Guide [eBook] GMP Compliance of Foreign Suppliers: The Importers Guide [eBook]](https://www.intouch-quality.com/hs-fs/hubfs/enforcement%20mechanisms.jpg?width=666&name=enforcement%20mechanisms.jpg)