The In vitro diagnostic medical devices regulation (EU) 2017/746: what will change for public health

European in Vitro Diagnostic Devices Regulation (EU) 2017/746 Tickets, Wed, Oct 11, 2023 at 10:00 AM | Eventbrite

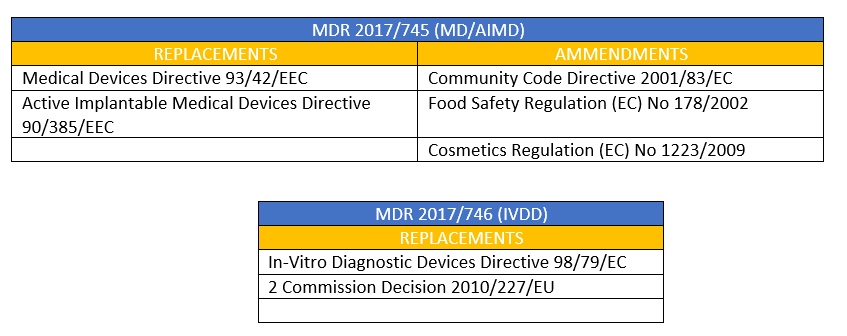
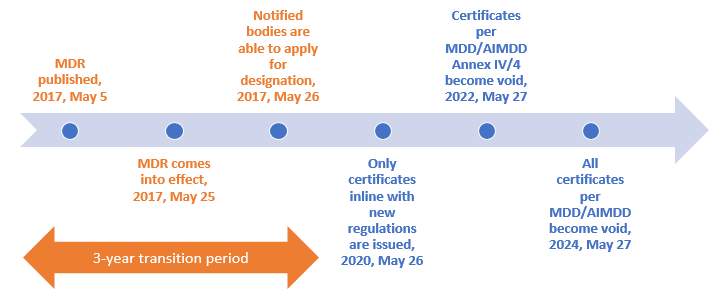
Reglamentos: Publicada la propuesta de reglamento modificando MDR e IVDR con la ampliación de los periodos transitorios
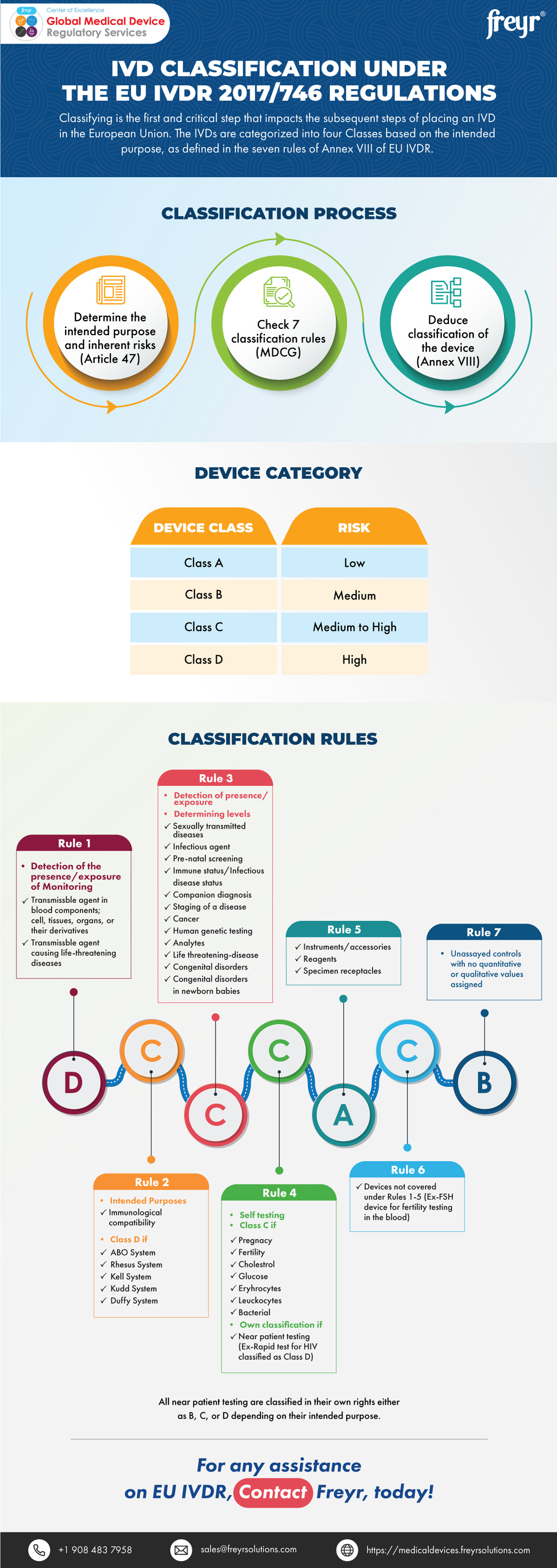
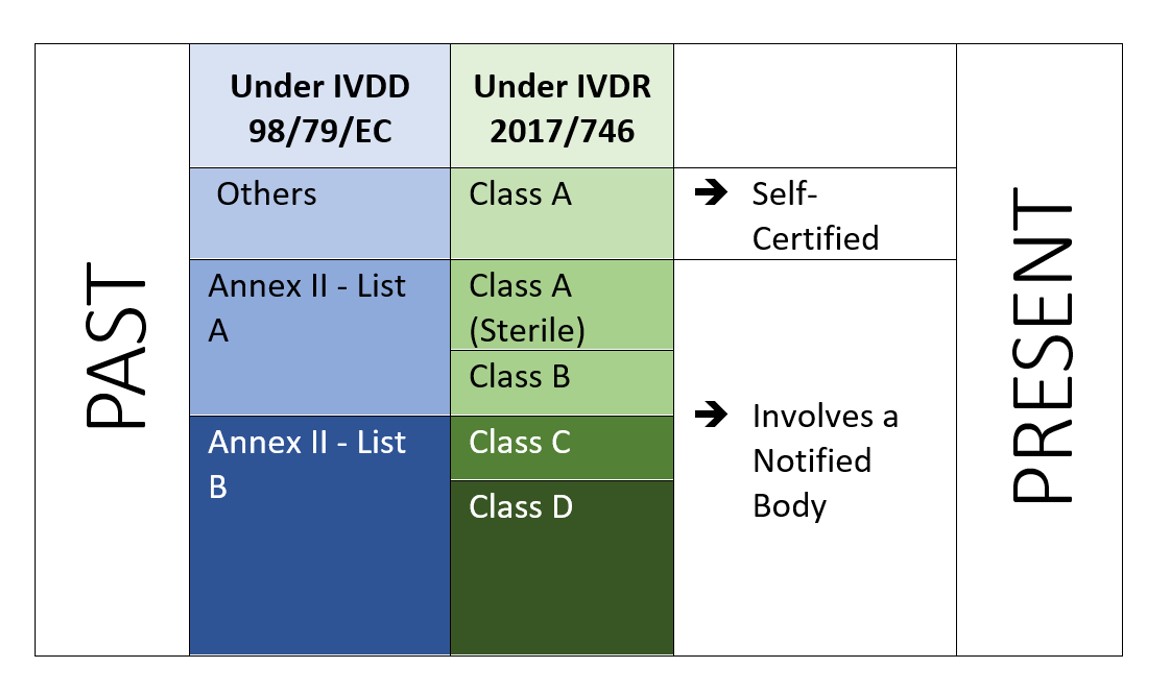
IVD Classification under the EU IVDR 2017/746 Regulations | Freyr - Global Regulatory Solutions and Services Company
Questions & Answers for applicants, marketing authorisation holders of medicinal products and notified bodies with respect t
Preparation is key: brief checklist how to bring IVD MD into compliance with EU IVDR - Biotech Spain

Application request for CE marking certification – Regulation (EU) 2017/746 - GMED Medical Device Certification

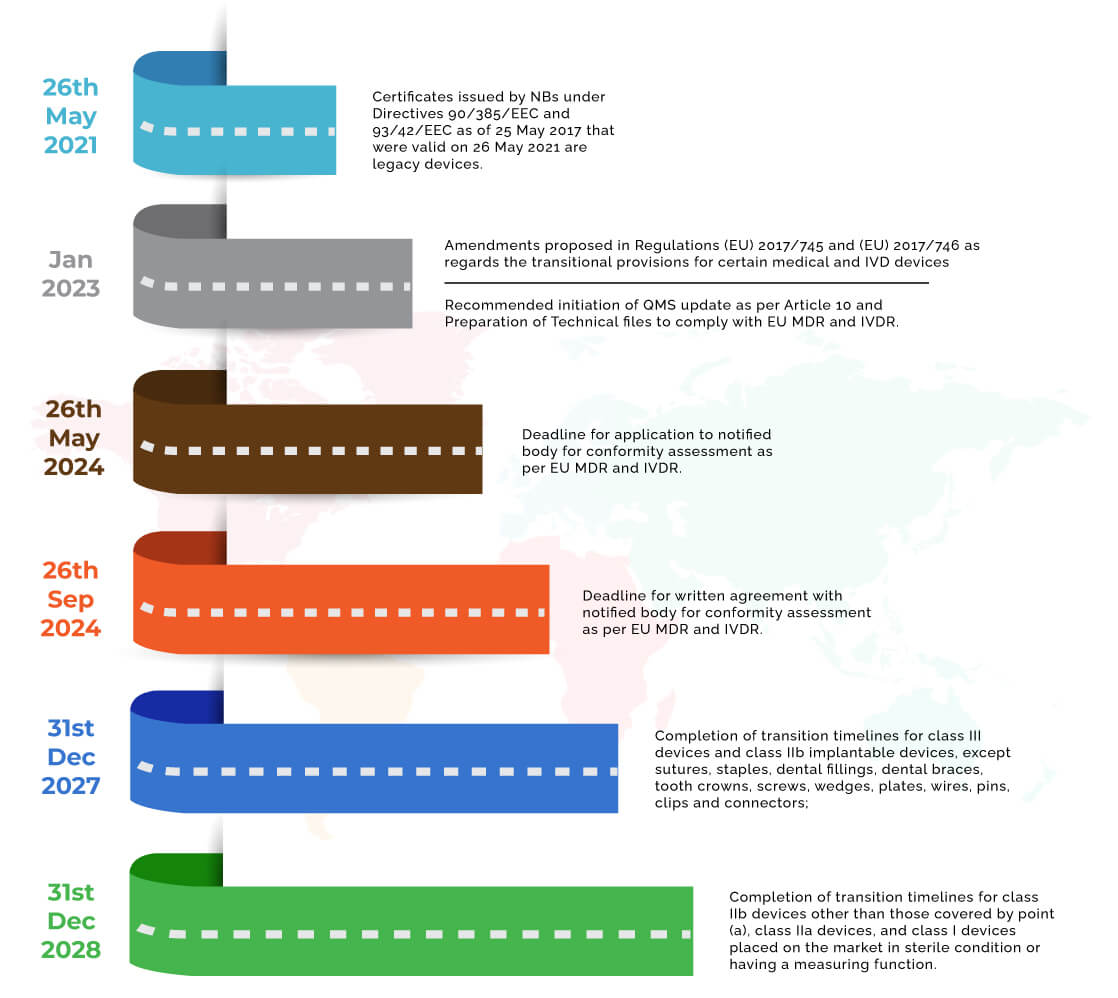
EU: Regulation 2017/746 — Amended transitional provisions for certain in vitro diagnostic medical devices - Global Compliance News