GCP and Quality in “Regulation (EU) 536/2014 on clinical trials on medicinal products for human use and repealing Directive 2001/20/EU” - ScienceDirect
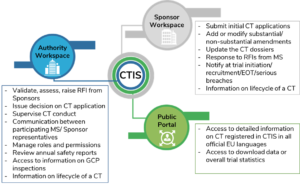
Adapting to the Evolving European Clinical Trial Regulatory Scenario: An Overview of the Current State of the European Clinical Trials Regulation and Clinical Trials Information System - ACRP

Clinical Evaluation and Investigation of Medical Devices under the new EU- Regulation : Ecker, Wolfgang, Labek, Gerold, Mittermayr, Tarquin, Raffeiner, Brigitte, Ring, Michael, Schwartz, Bernhard: Amazon.es: Libros

The new Clinical Trials Regulation – what you need to know now - Episode 1: Transitional period and timeline | Hogan Lovells - JDSupra
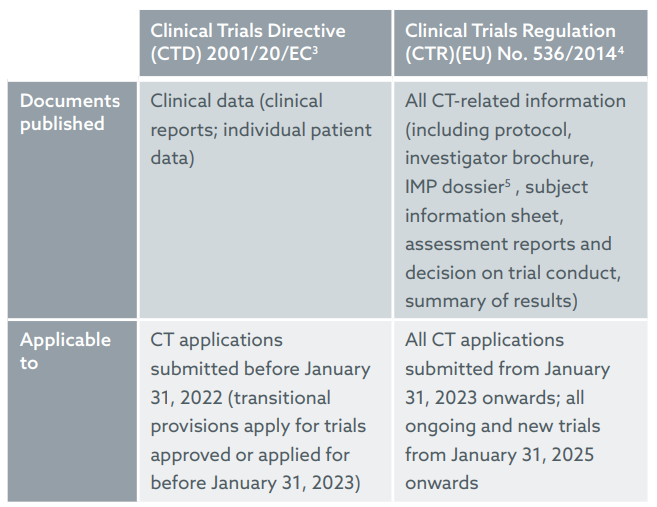
The EU Clinical Trials Regulation: Implications of the New Transparency Rules on Patenting - Lexology