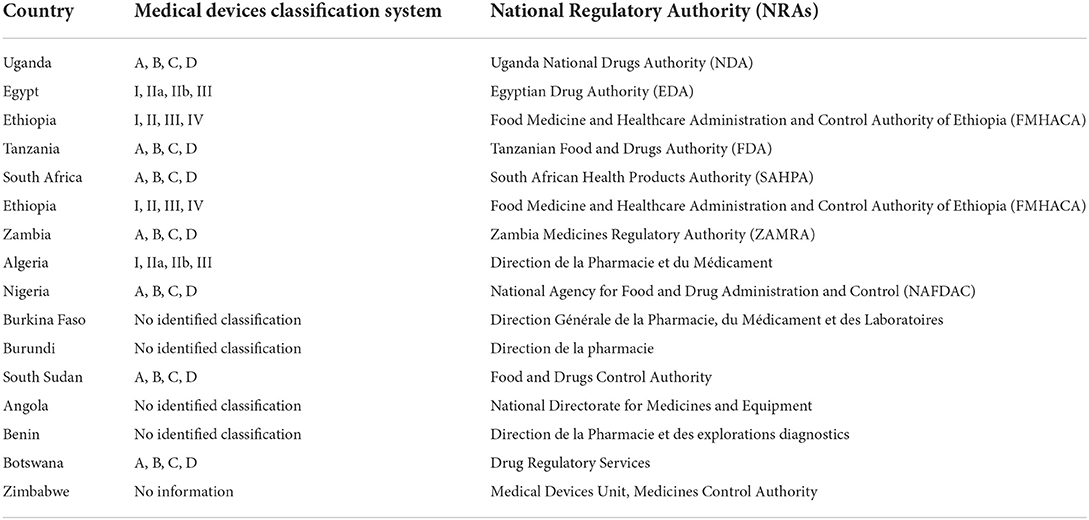
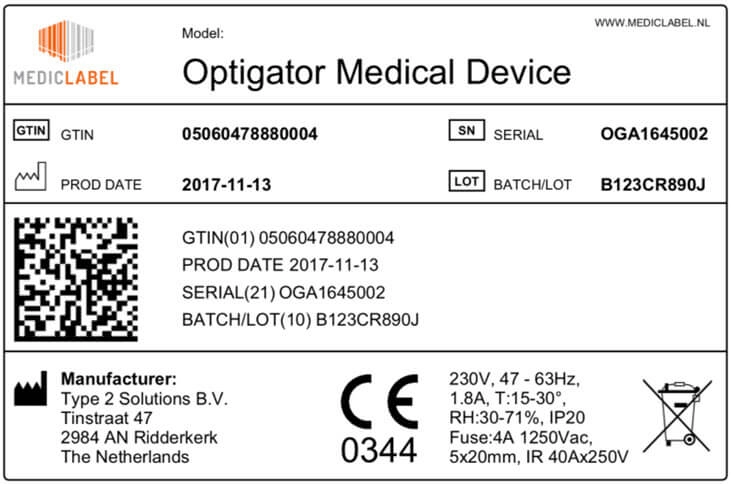
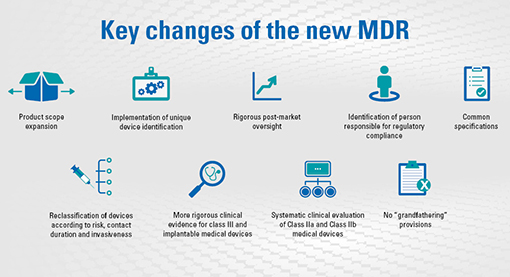
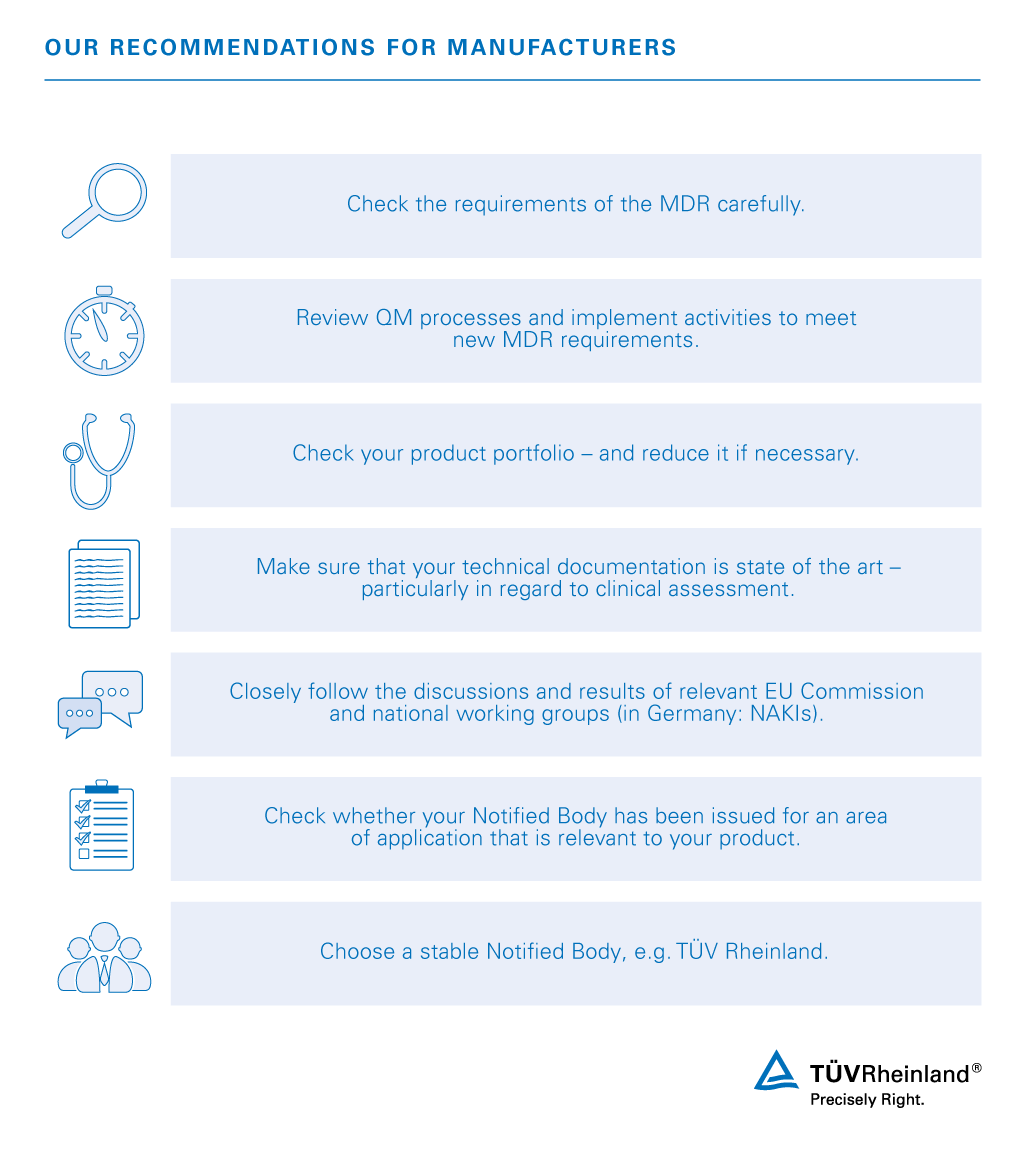
PDF) Accessing the Medical Devices Market in Egypt and Saudi Arabia: A Systematic Review of Policies and Regulations

PDF) Accessing the Medical Devices Market in Egypt and Saudi Arabia: A Systematic Review of Policies and Regulations

From Documentation to Market Approval: A Guide to Medical Device Registration Egypt | Operon Strategist