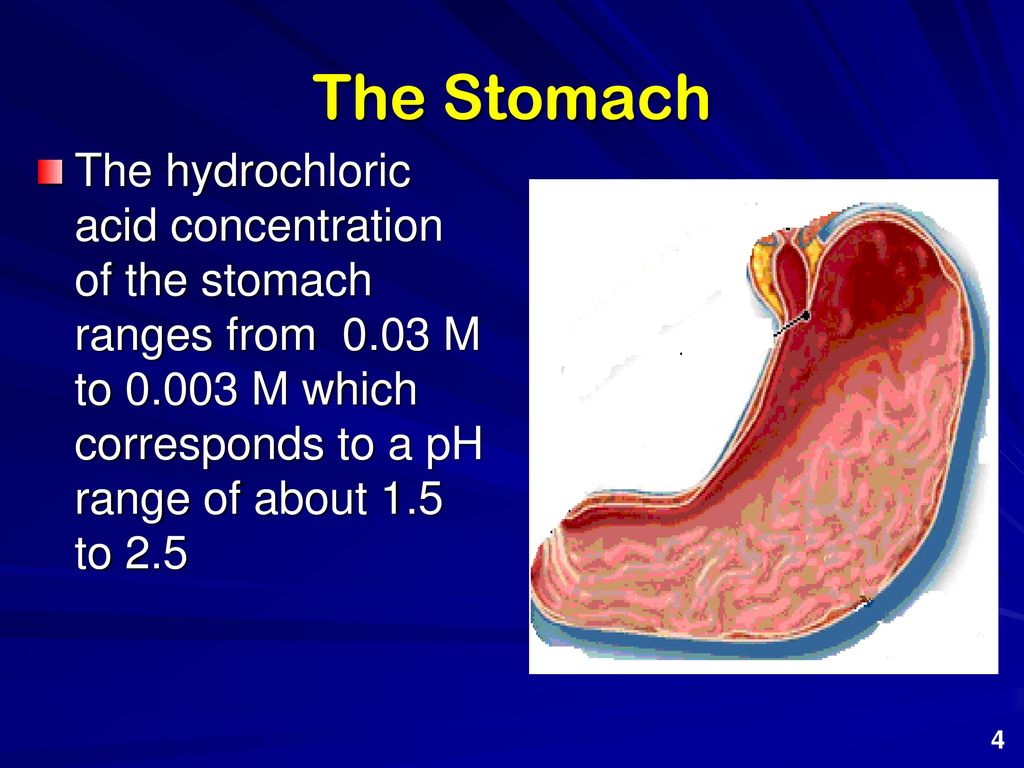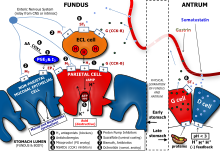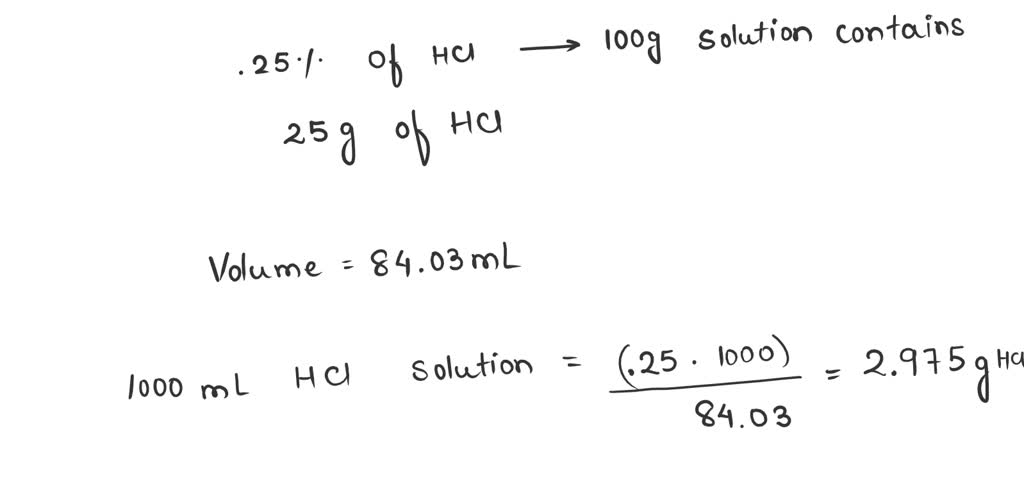
SOLVED: Given that stomach acid is 0.25% HCl by mass and has a density of 1.19 g/mL, determine whether the concentration of HCl (0.003 moles, 0.6 Molarity) in your sample is greater

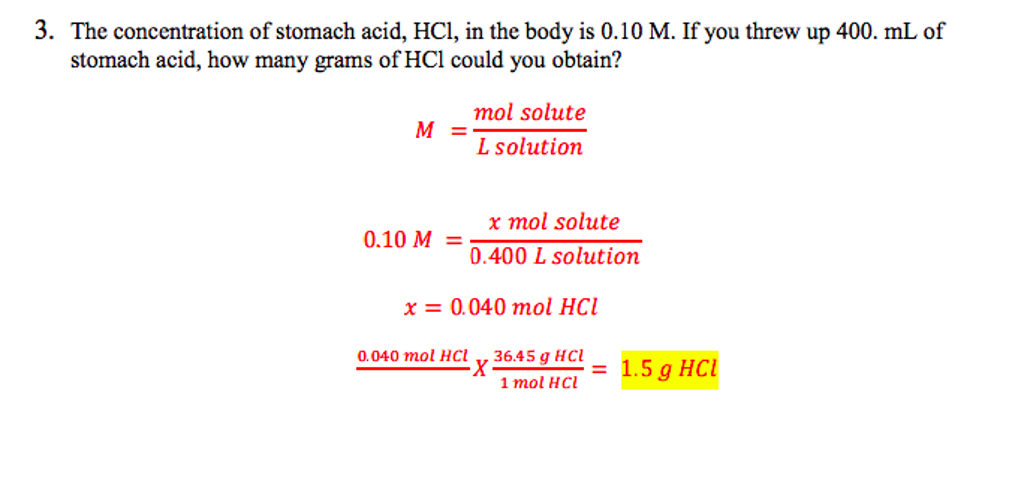
SOLVED: The concentration of stomach acid, HCl, is approximately 0.10 M . What volume of stomach acid contains 0.25 mg of HCl?
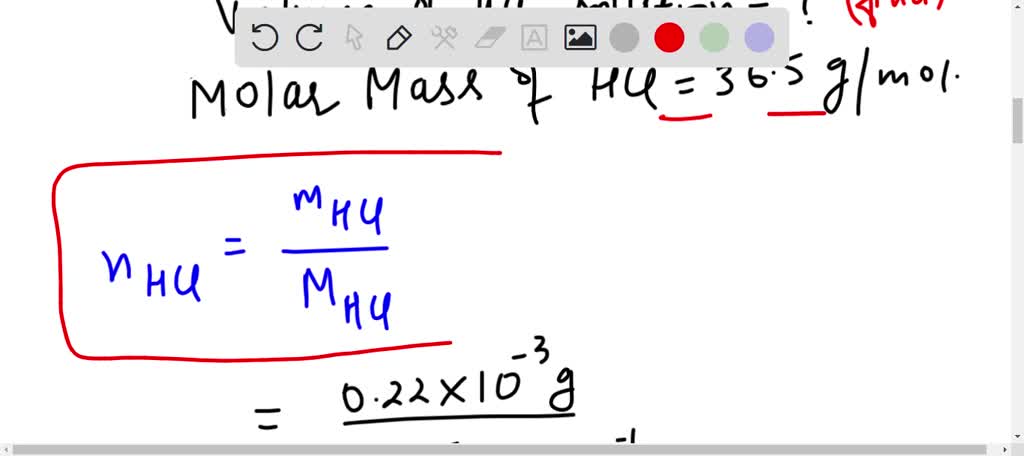
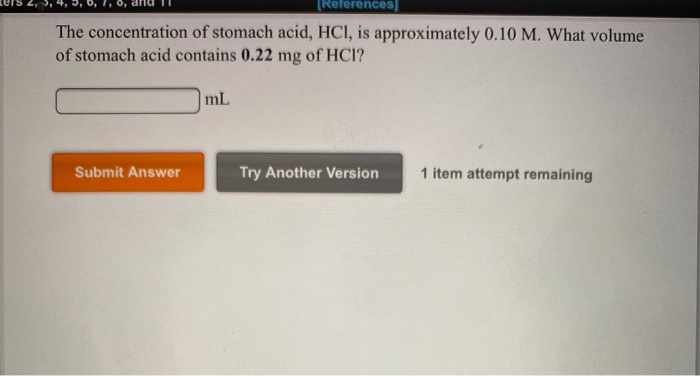
SOLVED: Chap6. The concentration of stomach acid, HCl, is approximately 0.10 M. What volume of stomach acid contains 0.22 mg of HCl?

Know Your Body: Is the stomach acid so strong that it can actually dissolve metal? | Health News - The Indian Express
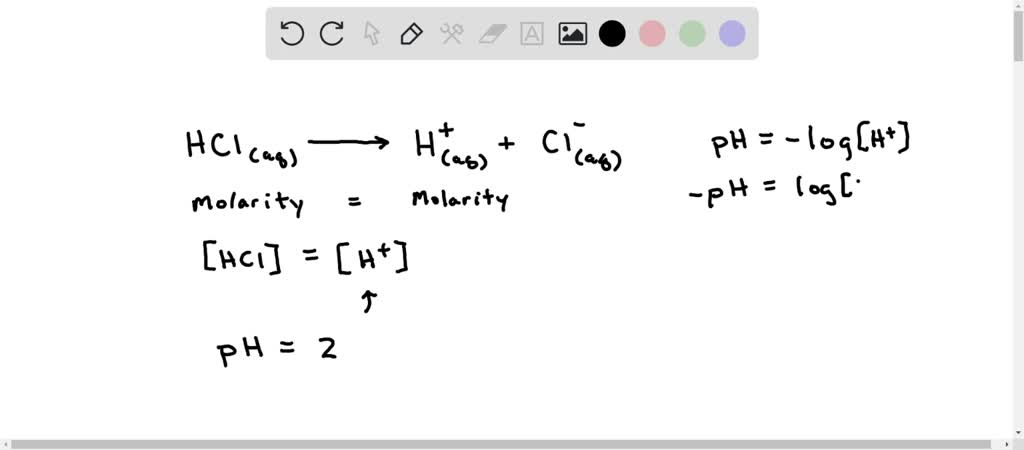
SOLVED: Normal gastric juice has a pH of about 2. Assuming that normal gastric juice is primarily aqueous HCI, what is the concentration of HCI in the stomach?
How many grams of Mg(OH) 2 will be needed to neutralize 25 mL of stomach acid if stomach acid is 0.10 M HCl? - Quora

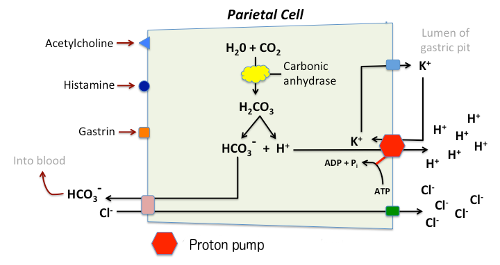
Determination of the concentration of acid in gastric juice - Determination of the concentration of - Studocu

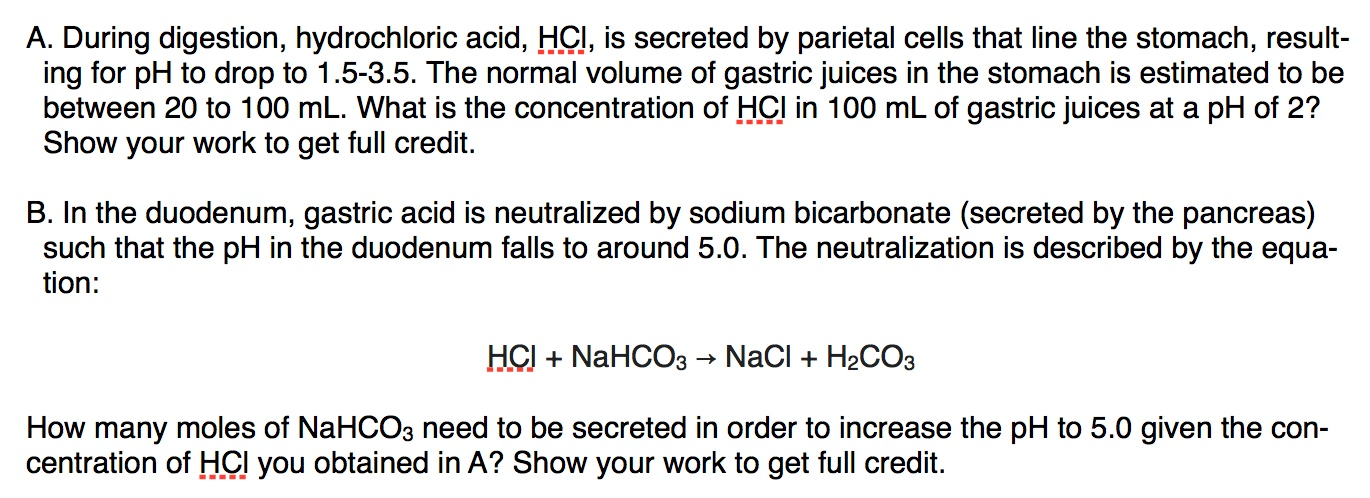
Stomach acid, a dilute solution of HCl in water can be neutralised by reaction with sodium hydrogen carbonate. NaHCO3(aq) + HCl(aq)⟶ NaCl(aq) + H2O(l) + CO2(g) How many millilitres of 0.125 M

SOLVED:The typical concentration of hydrochloric acid in stomach acid (digestive juice) is about 8.0 ×10^-2 M. One experiences "acid stomach" when the stomach contents reach about 1.0 ×10^-1 M HCl. One antacid

Gastric juice contains 3 g of HCl per litre. If a person produces 2.5 litres of gastric juice per day, how many antacid tablets each containing 400 mg of Al(OH)3 are needed

The appropriate concentration of HCL, in the stomach (stomach acid) is 0.17M , calculate the... - Myschool