EU Medical Device Regulation (EU MDR) – Chapter 10 – Final Provisions - LearnGxP: Accredited Online Life Science Training Courses
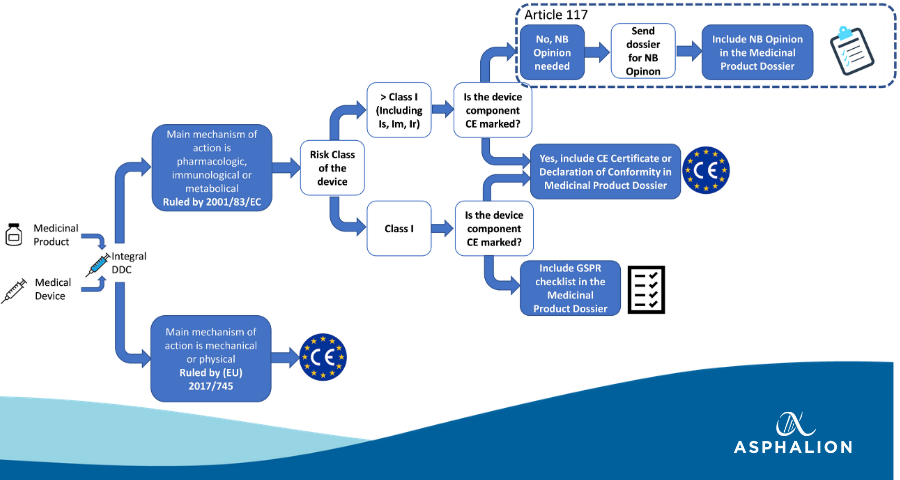
DEVICE REGULATIONS - The New Medical Device Regulation & the Applicability of Article 117 to Medicinal Products
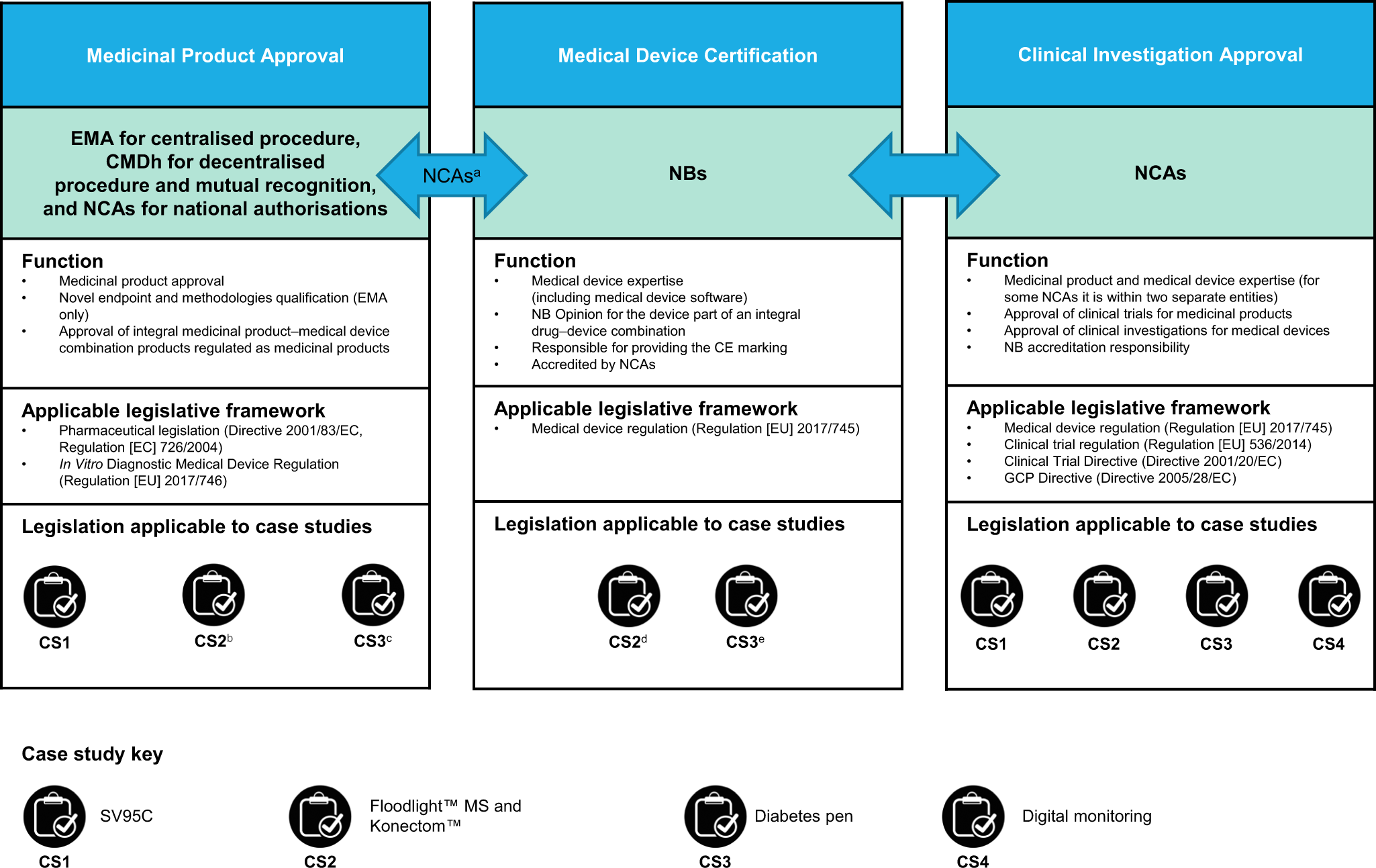
Evolving regulatory perspectives on digital health technologies for medicinal product development | npj Digital Medicine
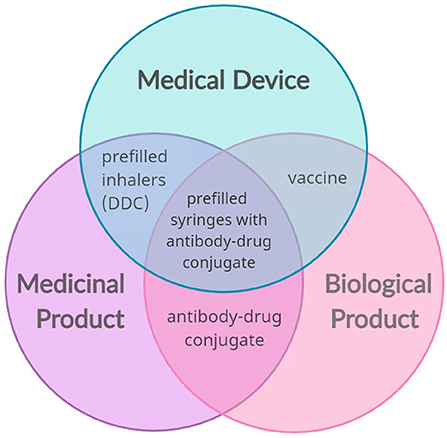
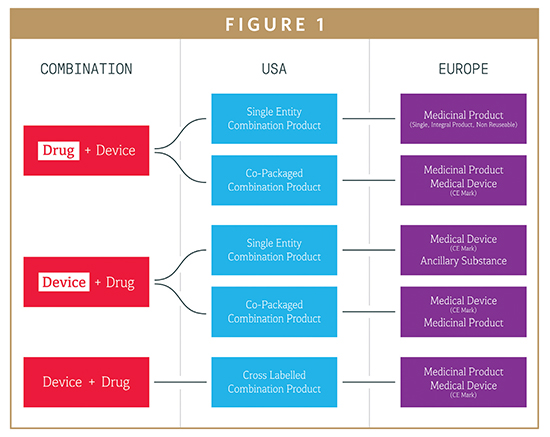
Exchange on the practical considerations for the future regulation of integrated drug- device combinations






![Medical Devices] BSI가 전세계 최초로 Article 117 Notified Body Opinion을 발행하였습니다 | BSI Blog Medical Devices] BSI가 전세계 최초로 Article 117 Notified Body Opinion을 발행하였습니다 | BSI Blog](http://bsiblog.co.kr/wp-content/uploads/Medical-Device.png)