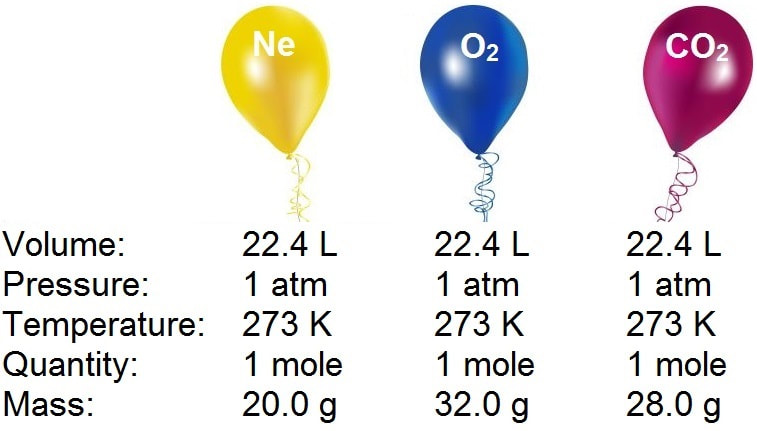
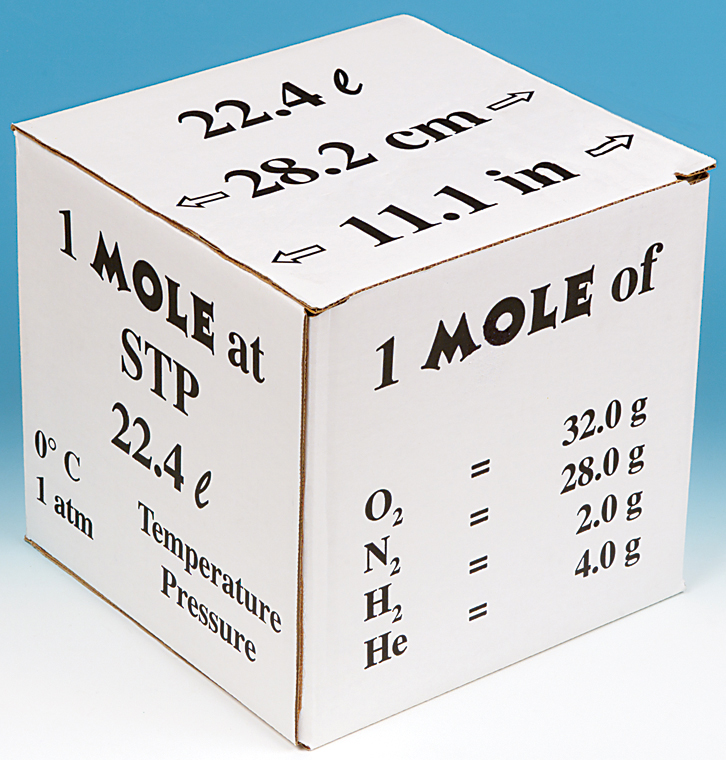
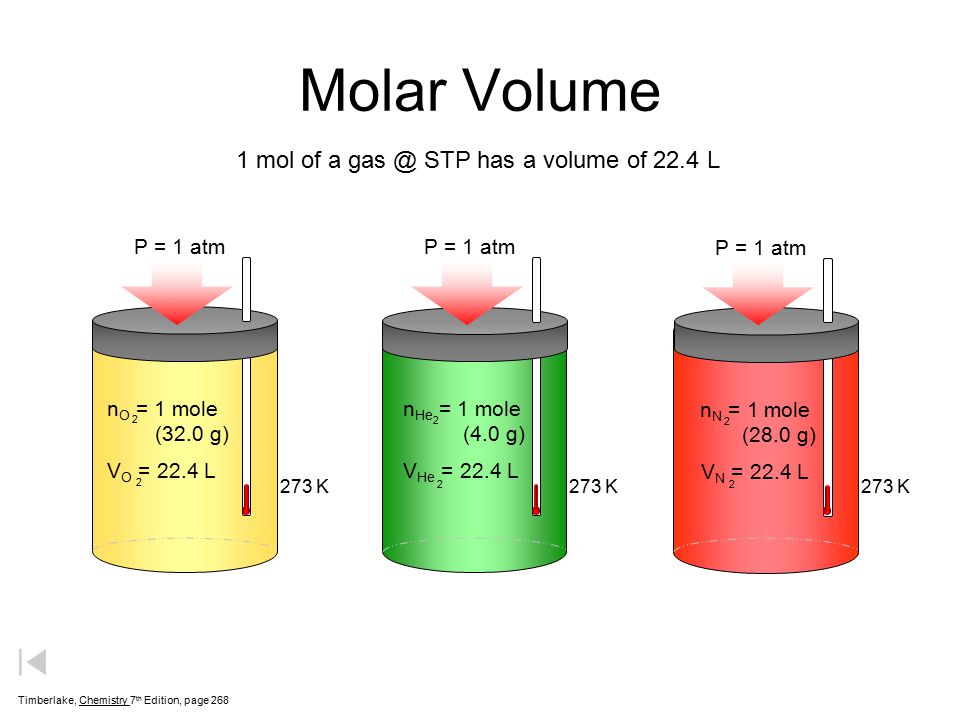
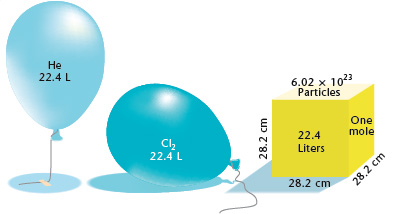
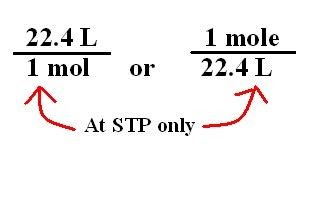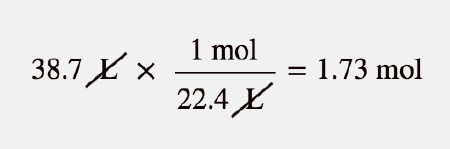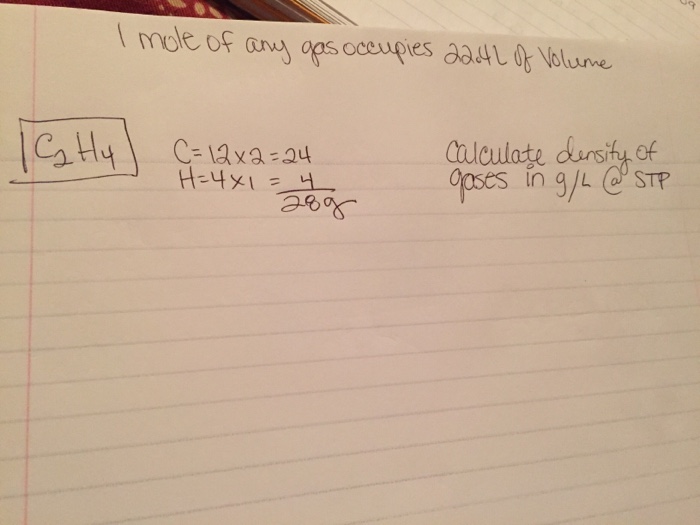If the volume of gas depends upon the size of container then why it is necessary that at STP one mole of a gas will have 22.4 litre of volume? - Quora
Assertion : 22.4L of He gas is having Avogadro number of He at 1atm.and 273k Reason : 22.4L of any gas at 1atm and 273k is having Avogadro number atoms

SOLVED: Calculate One mole of a gas occupies a volume of 22.4 L at STP. Calculate the temperature and pressure conditions needed to fit 2 mol of a gas into a volume

Gases & Stoichiometry. Molar Volume 1 mol of gas = 22.4 L molar volume What volume would be occupied by 0.77 moles of helium gas at STP? - ppt download
At 0°C, a gas occupies 22.4 liters. How nuch hot must be the gas in celsius and in kelvin to reach volume of 25.0 literes? - Sarthaks eConnect | Largest Online Education Community
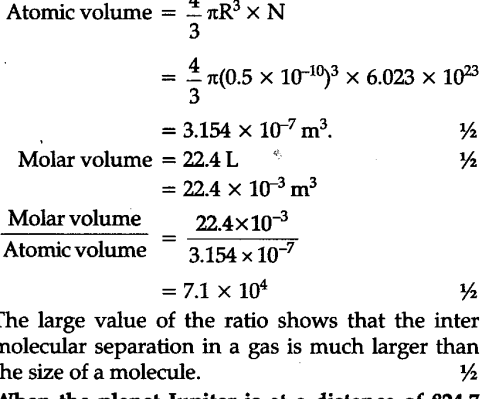
One mole of an ideal gas at NTP and pressure occupies 22.4 L (molar volume) - CBSE Class 11 Physics - Learn CBSE Forum